Answered step by step
Verified Expert Solution
Question
1 Approved Answer
kindly answer from a to f in a clear and understanding way Q3: Toluene (C/Hs) reacts with H, to form benzene (C.He desired product), but

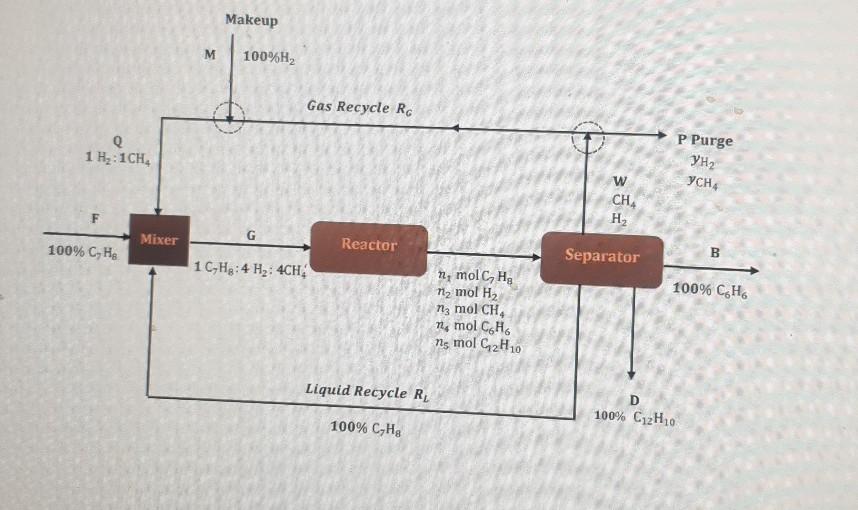
kindly answer from a to f in a clear and understanding way
Q3: Toluene (C/Hs) reacts with H, to form benzene (C.He desired product), but a side reaction occurs in which diphenyl (C12H10, undesired product), is formed. CHg C. Ho 1 + CHA 2C,H, + H2 C1H0 t 2CH CHENG 111 - Introduction to Chemical Engineering 2021-22 Sem 2 The process is shown in the figure below. Hydrogen is added to the gas recycle stream to make the ratio of H2 to CH4 1 to 1 before the gas enters the mixer. The ratio of H2 to toluene entering the reactor at G is 4 H2 to 1 toluene. The single-pass conversion of toluene is 88%, and the yield of benzene is 0.80. Based on 100 moles of toluene entering the reactor, calculate the following: a) molar composition of the reactor effluent using the extent of reaction approach. b) amount of gas recycle stream. c) molar composition of the purge stream. d) selectivity. e) overall conversion of toluene. f) Which reaction is predominating? Is it the main reaction or the side reaction? Why? Makeup M 100%H2 Gas Recycle RG P Purge Q 1 H:1CH YCH w CH F H Mixer G 100% C, H. Reactor Separator B 1 C-H3:4 Hz: 4CH 100% CH n, molc, Hg 11, mol H2 n3 mol CH, Me mol CH ns mol CH 10 Liquid Recycle RL D 100% CH10 100% C,HgStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


