Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Kindly derive all necessary equations for the multiple reactions happening in a Plug Flow Catalytic reactor. Solve the equations using RK4 method to get the
 Kindly derive all necessary equations for the multiple reactions happening in a Plug Flow Catalytic reactor.
Kindly derive all necessary equations for the multiple reactions happening in a Plug Flow Catalytic reactor.
Solve the equations using RK4 method to get the desired result (WEIGHT of catalyst when production of D is maximum)
Show all the calculations clearly.
If possible please attach excel screenshots of solving EQUATIONS USING RK4 along with the derived equations.
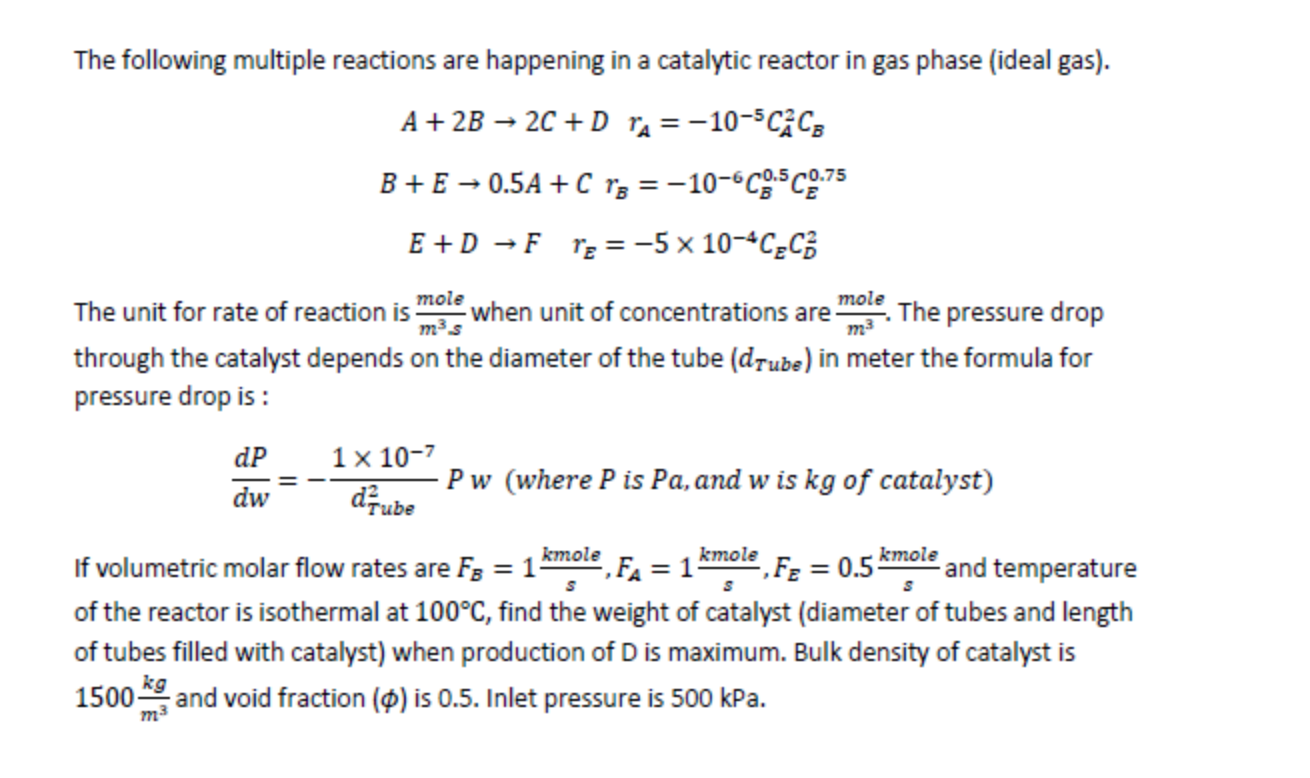
The following multiple reactions are happening in a catalytic reactor in gas phase (ideal gas). A+2B2C+DrA=105CA2CBB+E0.5A+CrB=106CB0.5CE0.75E+DFrE=5104CECD2 The unit for rate of reaction is m3smole when unit of concentrations are m3mole. The pressure drop through the catalyst depends on the diameter of the tube (dTube) in meter the formula for pressure drop is : dwdP=dTube21107Pw(wherePisPa,andwiskgofcatalyst) If volumetric molar flow rates are FB=1skmole,FA=1skmole,FE=0.5skmole and temperature of the reactor is isothermal at 100C, find the weight of catalyst (diameter of tubes and length of tubes filled with catalyst) when production of D is maximum. Bulk density of catalyst is 1500m3kg and void fraction () is 0.5 . Inlet pressure is 500kPaStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


