Answered step by step
Verified Expert Solution
Question
1 Approved Answer
l. [15 marks total] Consider a room whose dimensions are 5.00 metres by 4.00 metres by 2.50 metres (5.00m > 115 marks totall Consider a
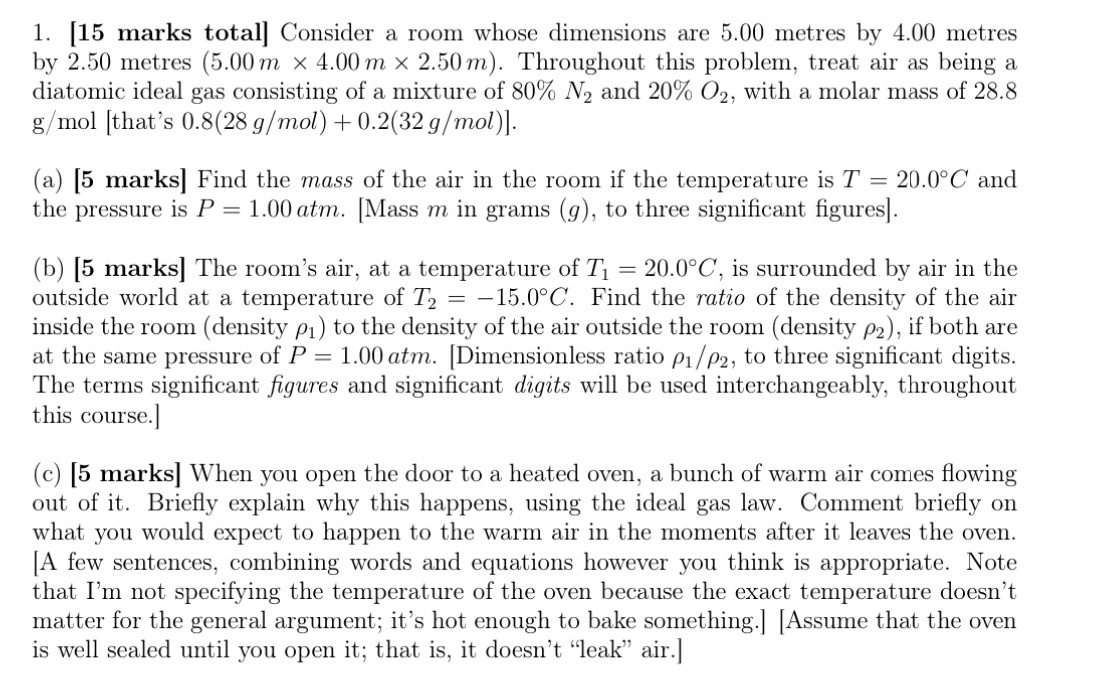
115 marks totall Consider a room whose dimensions are 5.00 metres by 4.00 metres 1. by 2.50 metres (5.00m x 4.00m > < 2.50m). Throughout this problem, treat air as being a diatomic ideal gas consisting of a mixture of 80% N2 and 20% 02, with a molar mass of 28.8 g mol [that's 0.8(28 g/mol) + 0.2(32 g/mol)l. (a) 15 marks] Find the mass of the air in the room if the temperature is T 20.00C and the pressure is P 1.00 atm. [Mass m in grams (g), to three significant figuresl. (b) 15 marksl The room's air, at a temperature of Tl 20.00C, is surrounded by air in the outside world at a temperature of T2 15.00C. Find the ratio of the density of the air inside the room (density Pl) to the density of the air outside the room (density P2), if both are at the same pressure of P = 1.00 atm. [Dimensionless ratio P1/P2, to three significant digits. The terms significant figures and significant digits will be used interchangeably, throughout this course.l (c) [5 marks] When you open the door to a heated oven, a bunch of warm air comes flowing out of it. Briefly explain why this happens, using the ideal gas law. Comment briefly on what you would expect to happen to the warm air in the moments after it leaves the oven. IA few sentences, combining words and equations however you think is appropriate. Note that I'm not specifying the temperature of the oven because the exact temperature doesn't matter for the general argument; it's hot enough to bake something.] [Assume that the oven is well sealed until you open it; that is, it doesn't "leak" air.]
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


