Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Lab question. or 3 mL less than the total volume added in the first trial) to save time. 12. Repeat steps 10 and 11. You
Lab question.
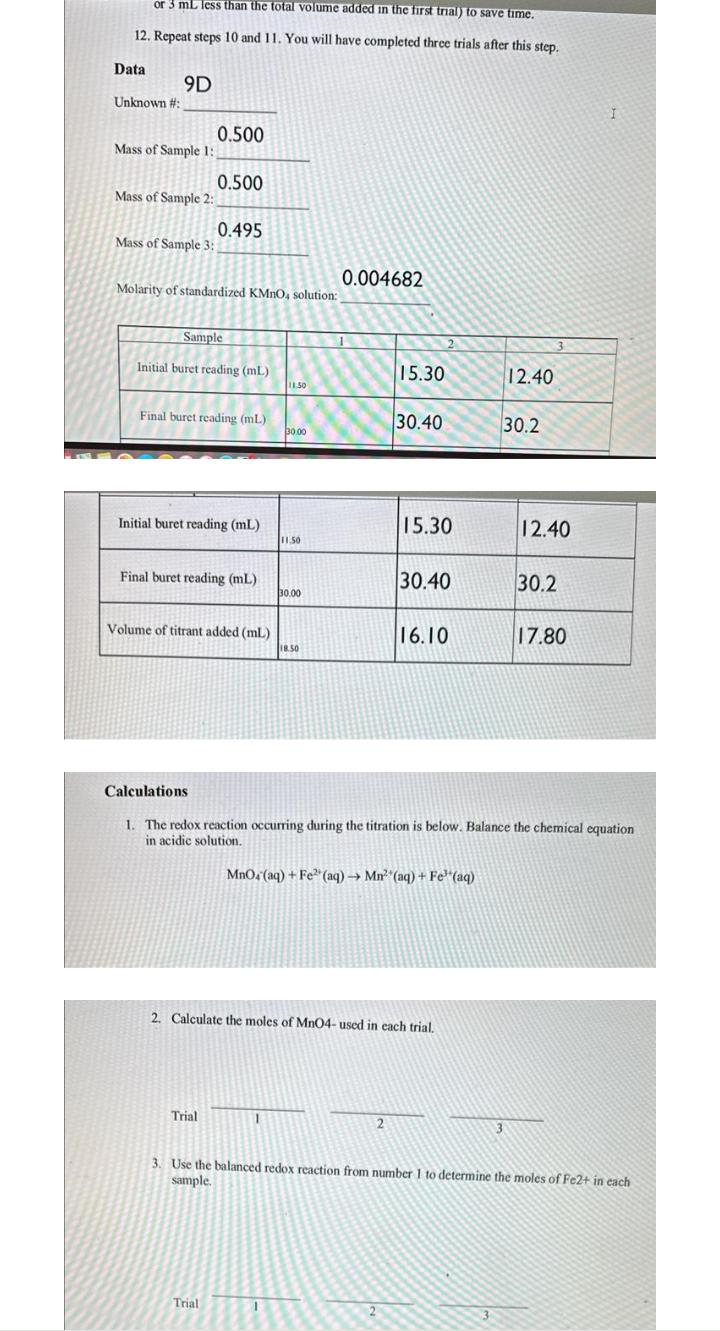

or 3 mL less than the total volume added in the first trial) to save time. 12. Repeat steps 10 and 11. You will have completed three trials after this step. Data Unknown #: 9D Mass of Sample 1: Mass of Sample 2: Mass of Sample 3: 0.500 0.500 Molarity of standardized KMnO, solution: 0.495 Sample Initial buret reading (mL) Final buret reading (mL) Initial buret reading (mL) Final buret reading (mL) Volume of titrant added (ml) Trial Trial 11.50 30.00 11.50 1 30.00 18.50 0.004682 1 15.30 30.40 15.30 2 30.40 2. Calculate the moles of MnO4- used in each trial. 16.10 MnO4 (aq) + Fe (aq) Mn (aq) + Fe (aq) 12.40 Calculations 1. The redox reaction occurring during the titration is below. Balance the chemical equation in acidic solution. 30.2 12.40 30.2 17.80 I 3. Use the balanced redox reaction from number 1 to determine the moles of Fe2+ in each sample.
Step by Step Solution
★★★★★
3.36 Rating (137 Votes )
There are 3 Steps involved in it
Step: 1
The detailed an...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


