Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Lab Report: Determination of Iron in Cereals (I need the answer and calculation for this experiment) Today's Experiment Absorbance 0.8 0.7 06 05 0.4 03
Lab Report: Determination of Iron in Cereals (I need the answer and calculation for this experiment)
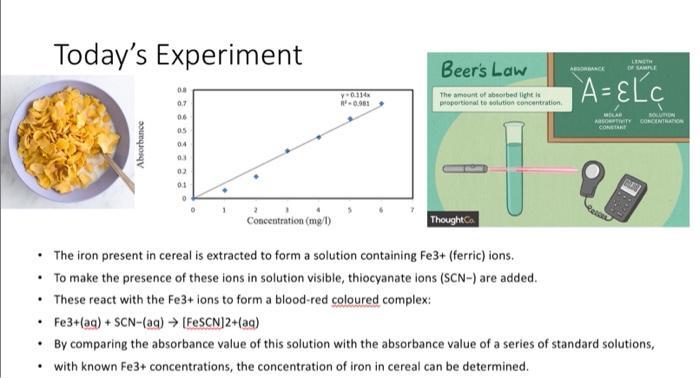
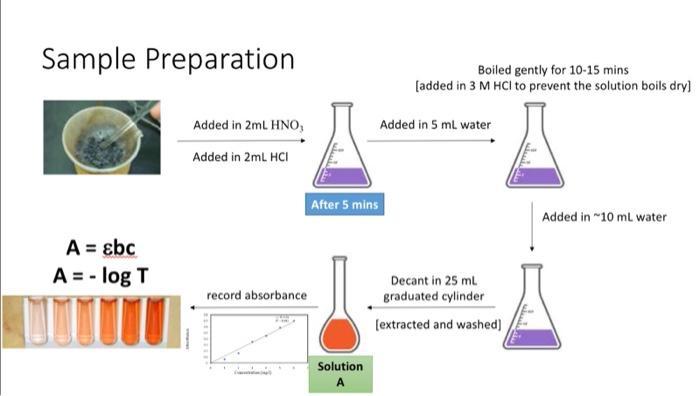
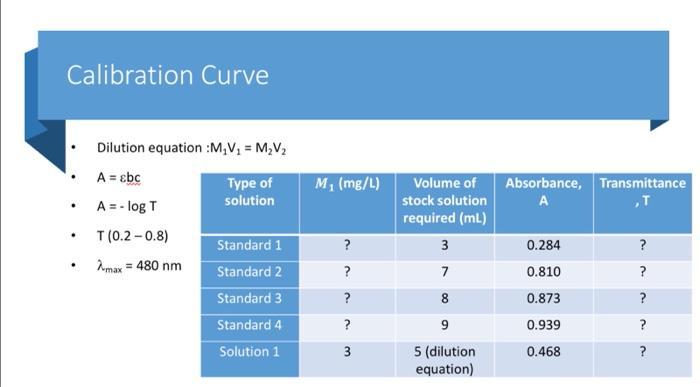


Today's Experiment Absorbance 0.8 0.7 06 05 0.4 03 02 01 0 Concentration (mg/l) y-0.114x R-0.961 Beer's Law The amount of absorbed light is proportional to solution concentration. ThoughtCo LENGTH OF SAMPLE A-ELC ABSORBANCE MOLAR SOLUTION ASOPIVITY CONCENTRATION CONSTAN The iron present in cereal is extracted to form a solution containing Fe3+ (ferric) ions. To make the presence of these ions in solution visible, thiocyanate ions (SCN-) are added. These react with the Fe3+ ions to form a blood-red coloured complex: Fe3+ (aq) + SCN- (aq) [FeSCN]2+(ag) By comparing the absorbance value of this solution with the absorbance value of a series of standard solutions, with known Fe3+ concentrations, the concentration of iron in cereal can be determined.
Step by Step Solution
★★★★★
3.33 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
Question 3 y115x 160 x feseN y A Sample absake x Fese 2 Soln A mlar cone 1492160 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


