Question
Lactic acid (C 3 H 6 O 3 ) produced by fermentation is used in the food, chemical, and pharmaceutical industries. Figure1 illustrates the mixing
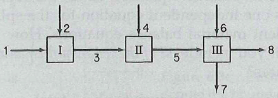
Lactic acid (C3H6O3) produced by fermentation is used in the food, chemical, and pharmaceutical industries. Figure1 illustrates the mixing of components to form a suitable fermentation broth. The whole system is steady-state and open. The arrows designate the direction of the flows. No reaction occurs in any of the subsystems.
The mass compositions of each stream are as follows:
1. Water (W): 100%
2. Glucose (G): 100%
3. W and G, concentrations known: W = 0.800 and G = 0.200
4. Lactobacillus (L): 100%
5. W, G, and L, concentrations known: W = 0.769, G = 0.192, L = 0.0385
6. Vitamin G with amino acids and phosphate (V): 100%
7. W = 0.962, V = 0.0385
8. G = 0.833, L = 0.167
- What is the maximum number of independent mass balances that can be generated for this system?
- What is the Flow rate of each stream entering and leaving the units I, II, and III?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


