Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Lam mol 1. Show your work for a full or a partial credit: the ideal gas law: PV = nRT where R=0.08206 The pressure needed
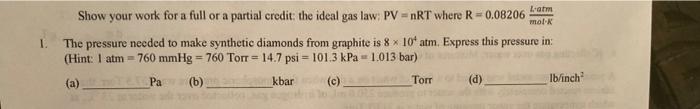 Lam mol 1. Show your work for a full or a partial credit: the ideal gas law: PV = nRT where R=0.08206 The pressure needed to make synthetic diamonds from graphite is 8 x 10' atm. Express this pressure in: (Hint: 1 atm = 760 mmHg = 760 Torr = 14.7 psi = 101.3 kPa 1.013 bar) (a) Pa (b) kbar (c) _ Torr (d) Ib/inch
Lam mol 1. Show your work for a full or a partial credit: the ideal gas law: PV = nRT where R=0.08206 The pressure needed to make synthetic diamonds from graphite is 8 x 10' atm. Express this pressure in: (Hint: 1 atm = 760 mmHg = 760 Torr = 14.7 psi = 101.3 kPa 1.013 bar) (a) Pa (b) kbar (c) _ Torr (d) Ib/inch
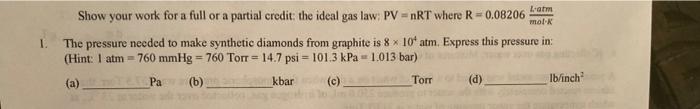
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


