Answered step by step
Verified Expert Solution
Question
1 Approved Answer
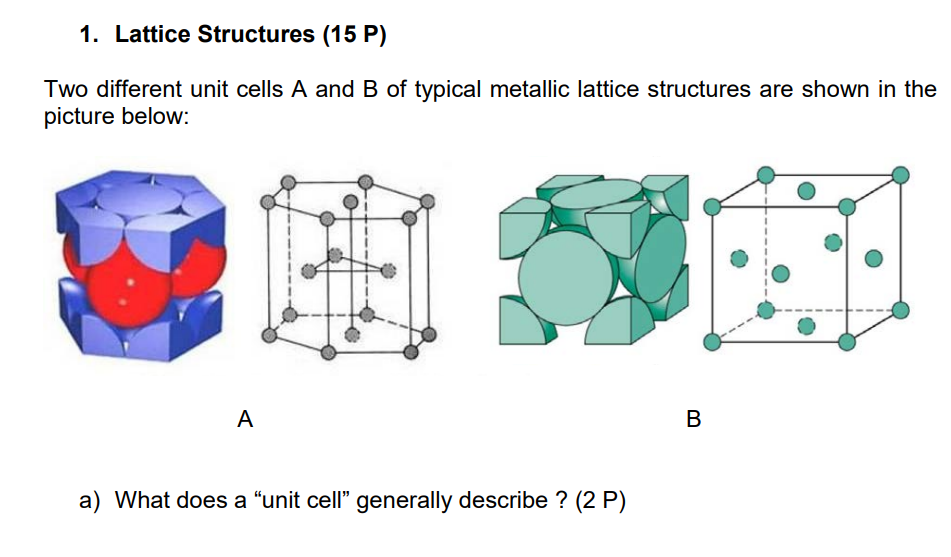
Lattice Structures ( 1 5 P ) Two different unit cells A and B of typical metallic lattice structures are shown in the picture below:
Lattice Structures P
Two different unit cells A and B of typical metallic lattice structures are shown in the
picture below:
A
B
a What does a "unit cell" generally describe P
b Which type of unit cell is shown in A resp. B P
c What is meant by the effective number of atoms per unit cellEN What is
EN for A resp. B P
d What is the mathematical relation between atomic radius r and edge length
a of above shown unit cell B
e What is meant by the APF of a lattice structure Briefly explain.
f Please calculate the APF of structure B
g The APF of unit cells A and B is equal. What is the structural difference between the two lattice types Briefly explain
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


