Answered step by step
Verified Expert Solution
Question
1 Approved Answer
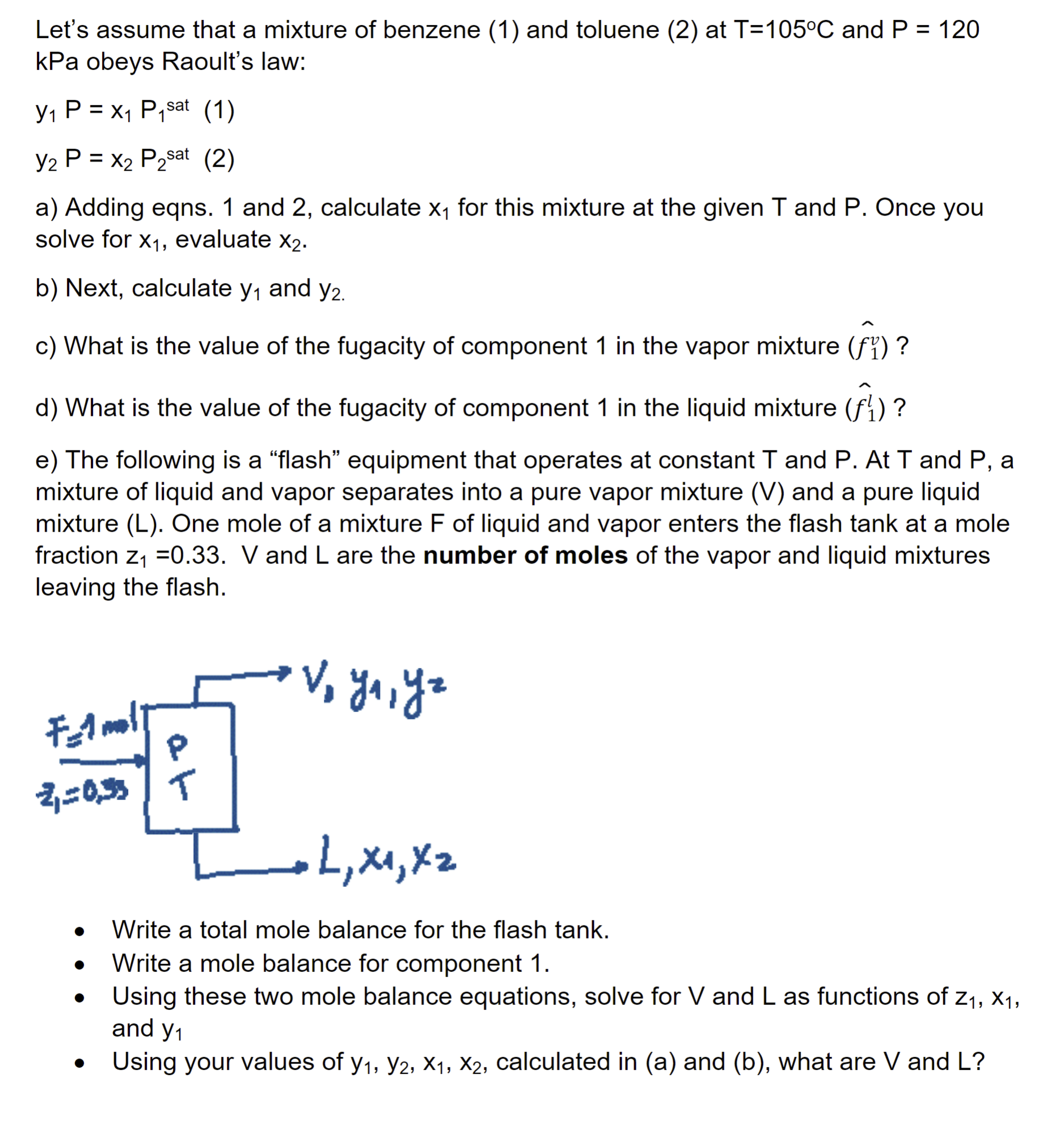
Let's assume that a mixture of benzene ( 1 ) and toluene ( 2 ) at T = 1 0 5 C and P =
Let's assume that a mixture of benzene and toluene at and
kPa obeys Raoult's law:
sat
sat
a Adding eqns. and calculate for this mixture at the given and Once you
solve for evaluate
b Next, calculate and
c What is the value of the fugacity of component in the vapor mixture
d What is the value of the fugacity of component in the liquid mixture
e The following is a "flash" equipment that operates at constant T and P At T and P a
mixture of liquid and vapor separates into a pure vapor mixture and a pure liquid
mixture One mole of a mixture of liquid and vapor enters the flash tank at a mole
fraction and are the number of moles of the vapor and liquid mixtures
leaving the flash.
Write a total mole balance for the flash tank.
Write a mole balance for component
Using these two mole balance equations, solve for and as functions of
and
Using your values of calculated in a and b what are and
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


