Answered step by step
Verified Expert Solution
Question
1 Approved Answer
In the lecture, we discussed the MO diagram for a square-planar AB4 molecule (shown on the right). The central atom A has s, p,
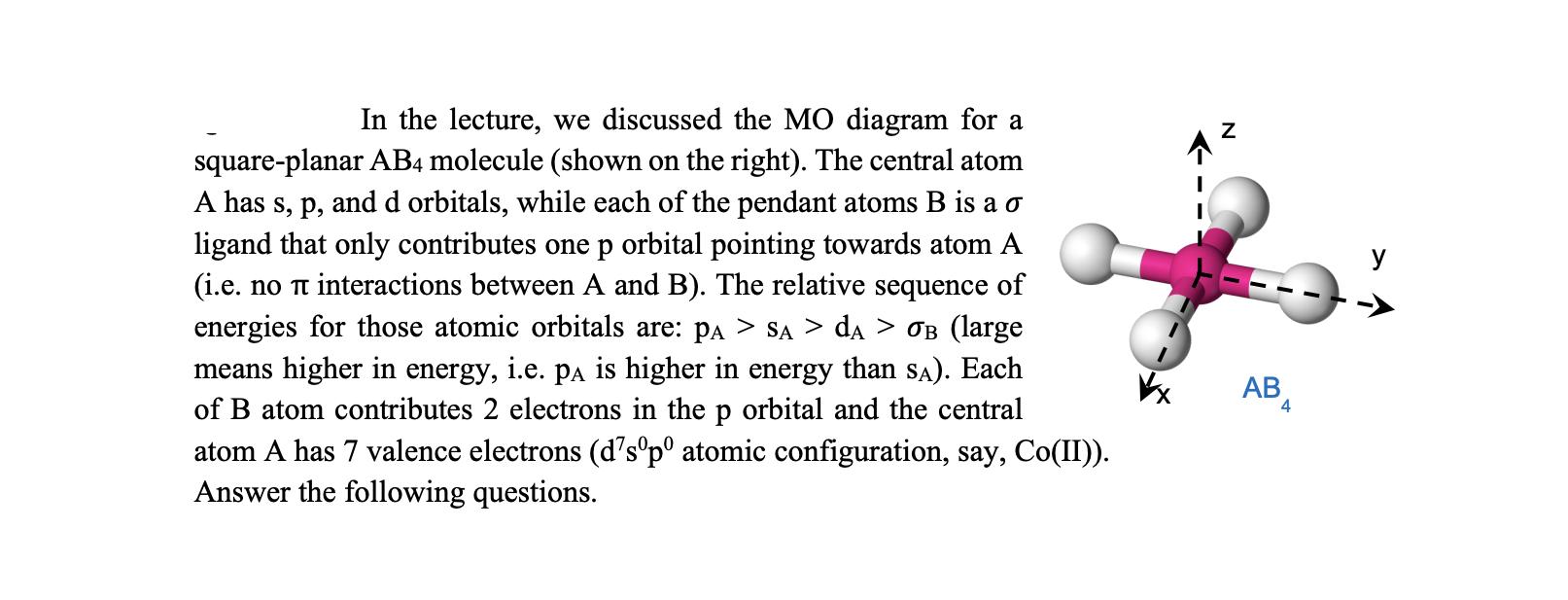

In the lecture, we discussed the MO diagram for a square-planar AB4 molecule (shown on the right). The central atom A has s, p, and d orbitals, while each of the pendant atoms B is a ligand that only contributes one p orbital pointing towards atom A (i.e. no interactions between A and B). The relative sequence of energies for those atomic orbitals are: PA > SA > dA > B (large means higher in energy, i.e. pA is higher in energy than SA). Each of B atom contributes 2 electrons in the p orbital and the central atom A has 7 valence electrons (d'sp atomic configuration, say, Co(II)). Answer the following questions. Z AB 4 1. Write down the point group of this molecule. 2. Write down the irreducible representations for the s. p, and d orbitals from A atom. 3. Derive and draw the SALC's for the four p orbitals for sigma interaction from ligand B. 4. Draw a schematic of the MO diagram for the above-stated molecule based on the symmetry. Please make your best effort to accurately position the relative energies for every MO. Label the irreducible representations for every MO. 5. Determine the bond order between each A-B interaction. 6. Write down the MOs in AB4 that are mostly characteristic of atom A's d orbitals. 7. Determine the HOMO of AB4 molecule (HOMO: highest occupied molecular orbital). 8. Derive all the symmetry-allowed optical excitations from the HOMO-1, if there is any. (HOMO: highest occupied molecular orbital; HOMO-1: the second highest occupied molecular orbital; You must show how you reach the results).
Step by Step Solution
★★★★★
3.50 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


