Question
Liquid benzene at 303 K is mixed and dissolved continuously in liquid toluene which is at 373 K in molar proportion 3:2 in an insulated
Liquid benzene at 303 K is mixed and dissolved continuously in liquid toluene which is at 373 K in molar proportion 3:2 in an insulated mixing tank. If the heat of mixing is zero, calculate the temperature of the final solution. Heat capacity of benzene liquid at 283 K=1.591 J/kg K, heat capacity of benzene liquid a 338 K = 2.018 J/kg K, heat capacity of Toluene liquid a 283 K=1.524 J/kg K, heat capacity of Toluene liquid at 358 K = 2.236 J/kg K. The datum temperature is 298 K. Heat capacity Benzene liquid and Toluene liquid varies with temperature as per the relationship Cp = a+bT, where a & b are constants and T is in K.
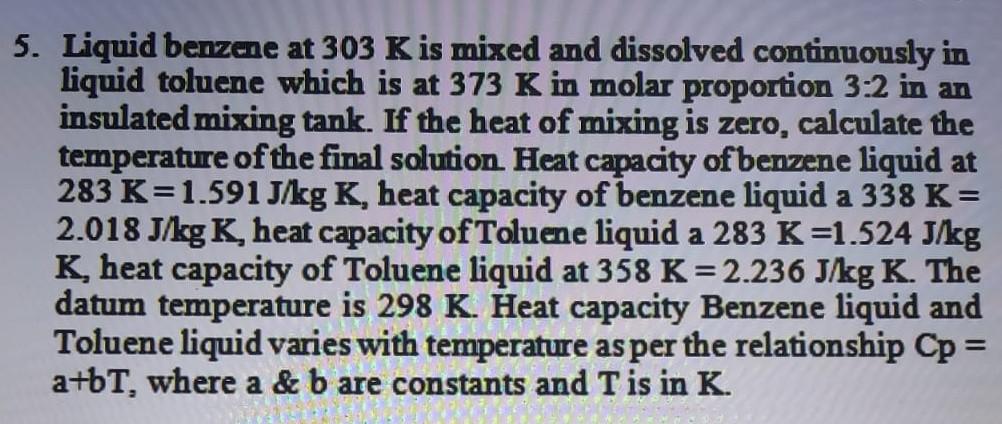
5. Liquid benzene at 303 K is mixed and dissolved continuously in liquid toluene which is at 373 K in molar proportion 3:2 in an insulated mixing tank. If the heat of mixing is zero, calculate the temperature of the final solution Heat capacity of benzene liquid at 283 K=1.591 J/kg K, heat capacity of benzene liquid a 338 K= 2.018 J/kg K heat capacity of Toluene liquid a 283 K=1.524 J/kg K, heat capacity of Toluene liquid at 358 K= 2.236 J/kg K. The datum temperature is 298 K. Heat capacity Benzene liquid and Toluene liquid varies with temperature as per the relationship Cp = a+bT, where a & b are constants and T is in K. =
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


