Question
LIST-I contains metal species and LIST-II contains their properties. LIST- I (P) (I) [Cr(CN)] (II) [RUCI, ] (III) [Cr(HO)]** (IV) [Fe(HO)]* LIST-II t2g orbitals
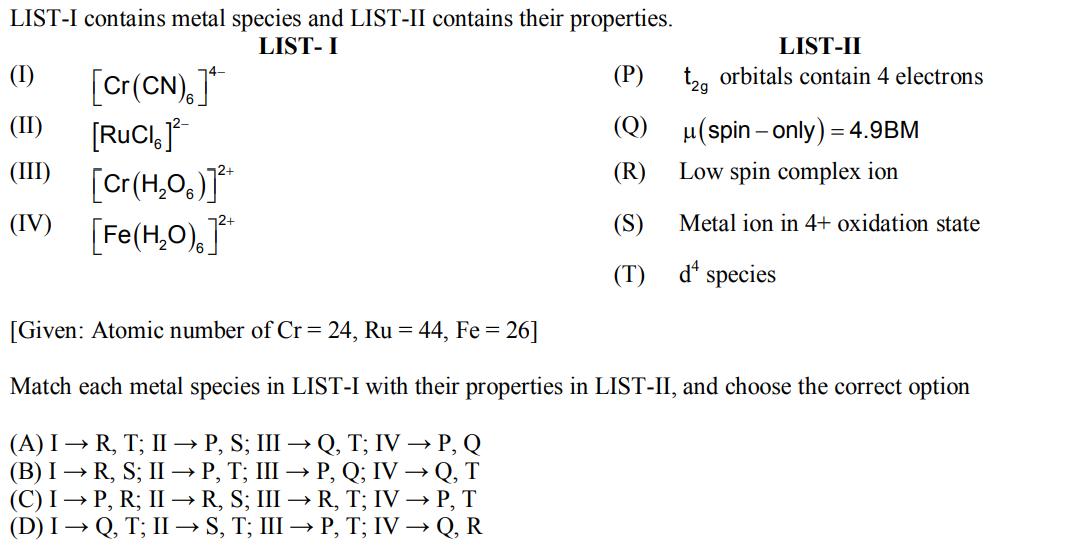
LIST-I contains metal species and LIST-II contains their properties. LIST- I (P) (I) [Cr(CN)] (II) [RUCI, ] (III) [Cr(HO)]** (IV) [Fe(HO)]* LIST-II t2g orbitals contain 4 electrons (Q)(spin-only) = 4.9BM Low spin complex ion Metal ion in 4+ oxidation state d species (R) (S) (T) [Given: Atomic number of Cr = 24, Ru = 44, Fe = 26] Match each metal species in LIST-I with their properties in LIST-II, and choose the correct option (A) I R, T; II P, S; III Q, T; IV P, Q (B) I R, S; II P, T; III P, Q; IV Q, T (C) I P, R; II R, S; III R, T; IV P, T (D) IQ, T; IIS, T; III P, T; IV Q, R
Step by Step Solution
3.37 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
The detailed answer for the abo...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Statistics For The Life Sciences
Authors: Myra Samuels, Jeffrey Witmer, Andrew Schaffner
5th Edition
321989589, 978-0321989581
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



