Answered step by step
Verified Expert Solution
Question
1 Approved Answer
m A 10-cm diameter pipe contains superheated gas maintained at a constant temperature of Ts = 600 C. If air at a temperature of
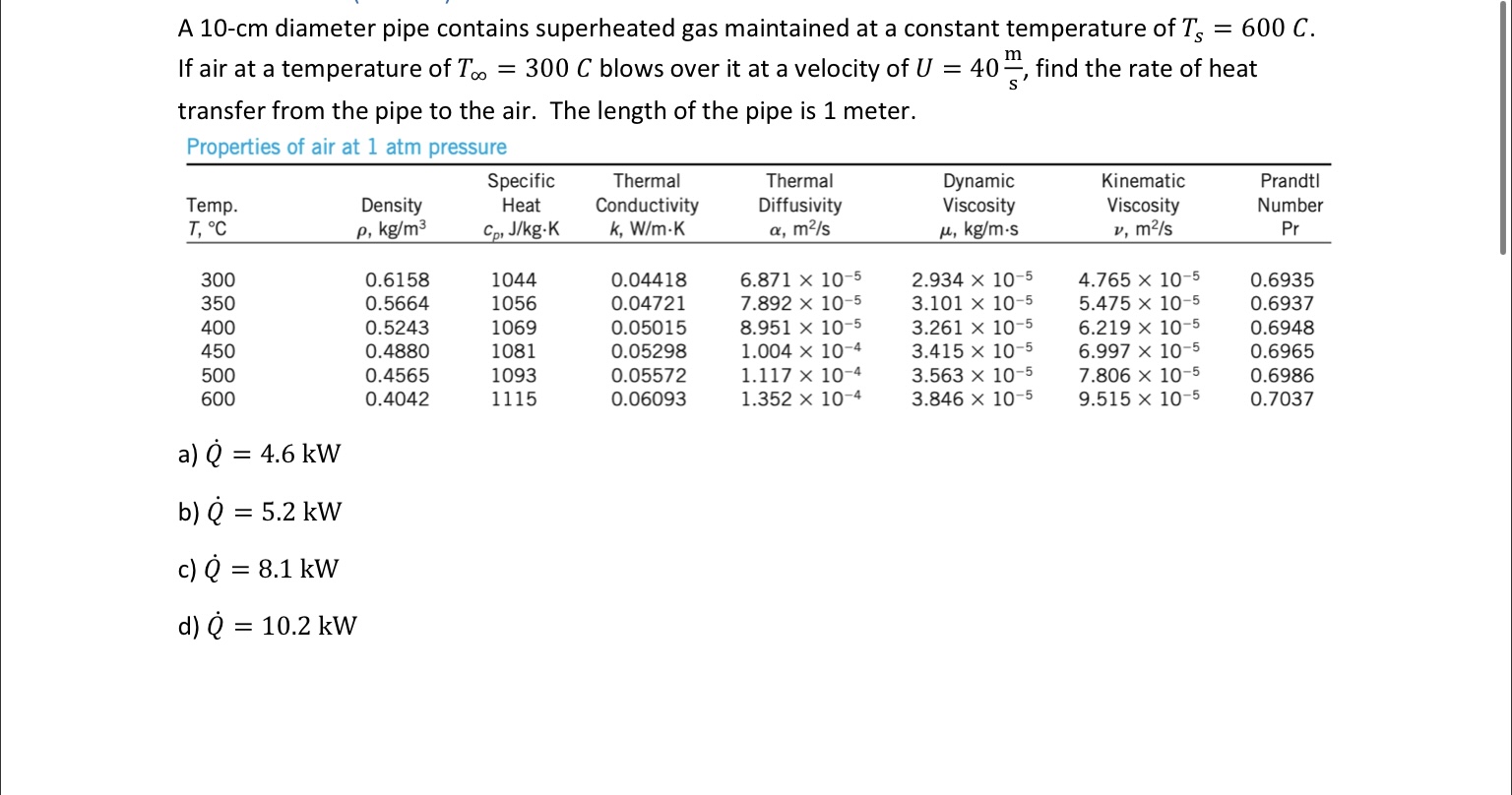

m A 10-cm diameter pipe contains superheated gas maintained at a constant temperature of Ts = 600 C. If air at a temperature of T.. = 300 C blows over it at a velocity of U = 40, find the rate of heat transfer from the pipe to the air. The length of the pipe is 1 meter. Properties of air at 1 atm pressure Temp. T, C 300 350 400 450 500 600 Density p, kg/m a) Q = 4.6 kW b) Q = 5.2 kW c) Q = 8.1 kW d) Q = 10.2 kW 0.6158 0.5664 0.5243 0.4880 0.4565 0.4042 Specific Heat Cp, J/kg-K 1044 1056 1069 1081 1093 1115 Thermal Conductivity k, W/m.K 0.04418 0.04721 0.05015 0.05298 0.05572 0.06093 Thermal Diffusivity , m/s 6.871 x 10-5 7.892 x 10-5 8.951 x 10-5 1.004 x 10-4 1.117 x 10-4 1.352 x 10-4 Dynamic Viscosity , kg/m-s 2.934 x 10-5 3.101 x 10-5 3.261 x 10-5 3.415 x 10-5 3.563 x 10-5 3.846 x 10-5 Kinematic Viscosity v, m/s 4.765 x 10-5 5.475 x 10-5 6.219 x 10-5 6.997 x 10-5 7.806 x 10-5 9.515 x 10-5 Prandtl Number Pr 0.6935 0.6937 0.6948 0.6965 0.6986 0.7037 Hot chocolate milk is piped through a 10-cm diameter pipe submerged in an ice bath (Ts = 0C) to cool it from Ti = 65C to Te = 5C. What is the average heat flux out of the milk if the milk is flowing at a speed of U = 0.8m? S kg W Use p = 1040 = 2.0 x 10-3. k 0.6 and Pr = 4.0. Assume the viscosity of the milk at the bulk temperature is approximately the same as at the surface temperature. kg ms' m' mk' a) q = 21 b) q = 35 kW m kW m kW m c) q = 39. kW m d) q = 47:
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


