Question
Magnetic Susceptibility acac complex n order to cause a reaction to occur according to the tri-molecular mechanism, it is necessary that after the iodine molecule
Magnetic Susceptibility
acac complex
n order to cause a reaction to occur according to the tri-molecular mechanism, it is necessary that after the iodine molecule breaks down into the iodine atoms, a reaction takes place (22 (and not a reaction)
A. Use a website to locate data on the reaction rate constant (22 (at different temperatures).
In the temperature range available in the data you found for this reaction, check the maximum temperature at which the experiment can be performed so that the initial velocity of a reaction (22) will be higher than the initial velocity of a reaction (3).
Instruction:
For speed calculations, use the appropriate velocity equation for each reaction.
The velocity constant of a reaction (3) must be calculated according to the data in Table 1.2.
The speed constant of response (22 (must be found on the website).
Use the initial concentrations of the materials you calculated in Assignment 1.
Note the units: Molecules should be switched to multiples by multiplying by the number
Avogadro.
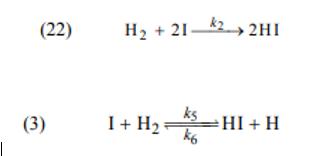
(22) (3) H +2122HI ks k6 HI+H 1 + H
Step by Step Solution
3.52 Rating (183 Votes )
There are 3 Steps involved in it
Step: 1
Answer Correct option is D 0216 109 J hc Energy given to I molecule 662...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


