Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Make sure to not follow the answers ob the internet or chegg itself because all of them are wrong. trust me, i tried it myself.
Make sure to not follow the answers ob the internet or chegg itself because all of them are wrong. trust me, i tried it myself. 

Construct the molecular orbital diagram for H2. Identify the bond order. 1.5 0.5 1 2 0 A Lewis structure is a two-dimensional representation of a molecule that does not necessarily show what shape that molecule would take in three dimensions. From the given Lewis structure and what you know about VSEPR theory, identify the shape of the molecule. linear T-shaped square pyramidal trigonal pyramidal octahedral tetrahedral trigonal planar see-saw bent square planar trigonal bipyramidal 1. 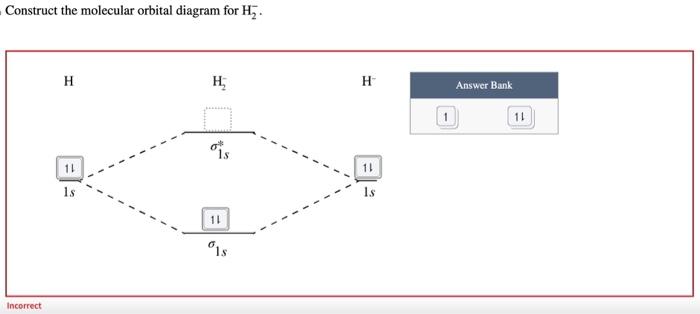


2.

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


