Answered step by step
Verified Expert Solution
Question
1 Approved Answer
mass of product obtained 2.466g and mass of substrate is 233.67 Questions: 1. Calculate (i) the theoretical yield and (ii) the % yield for your

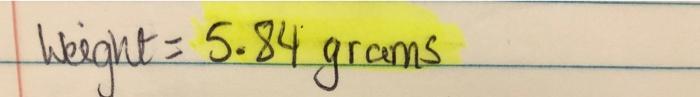

mass of product obtained 2.466g and mass of substrate is 233.67
Questions: 1. Calculate (i) the theoretical yield and (ii) the % yield for your product. Comment on the value. 2. Research your individual sulphonamide - background-history - uses and annlicatinne ata height = 5.84 grams SULFANILAMIDE Drug No. V P. p Transfer the moist sulfonyl chloride to the original 125-ml Erlenmeyer flask. Add 40 ml of concentrated aqueous ammonia and stir and shake. As the reaction proceeds, the granular sulfonyl chloride gives way to a thin paste of the amide. Warm for 5 minutes on the steam bath, then cool and collect the solid. Wash with water, press, and drain thoroughly. Transfer the cake of acetamidosulfonamide to the same Erlenmeyer flask and add 10 ml of 6N hydrochloric acid (5 ml concentrated HCl plus 5 ml water). Heat to boiling over a low flame until all solid has dissolved and continue gentle boiling for 10 minutes. Cool the solution and add water to replace that lost in evaporation; add activated carbon and filter through paper. The filtrate is then treated with solid sodium bicarbonate, with stirring, until the pH is neutral. Collect the solid sulfanilamide Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


