Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Mass of weighing cup and sodium carbonate Mass of weighing cup and residual sodium carbonate Mass of sodium carbonate used Temperature of solution before
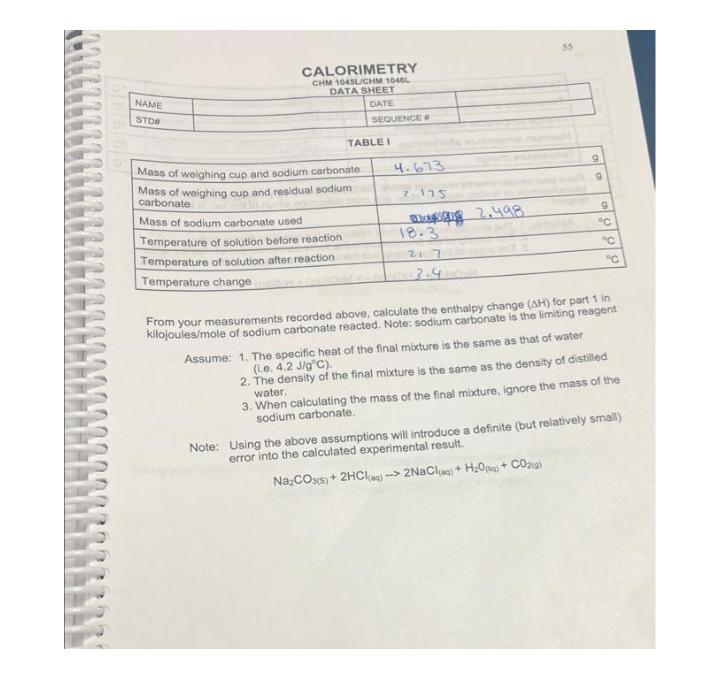
Mass of weighing cup and sodium carbonate Mass of weighing cup and residual sodium carbonate Mass of sodium carbonate used Temperature of solution before reaction Temperature of solution after reaction Temperature change 4.673 7.175 18.3 21.7 3.4 2.498 9 9 9 C C From your measurements recorded above, calculate the enthalpy change (AH) for part 1 in kilojoules/mole of sodium carbonate reacted. Note: sodium carbonate is the limiting reagent Assume: 1. The specific heat of the final mixture is the same as that of water (i.e. 4.2 J/gC). 2. The density of the final mixture is the same as the density of distilled water. 3. When calculating the mass of the final mixture, ignore the mass of the sodium carbonate. Note: Using the above assumptions will introduce a definite (but relatively small) error into the calculated experimental result. NaCO3(s) + 2HCl(aq)-> 2NaCl(aq) + HO + CO2(g)
Step by Step Solution
★★★★★
3.48 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1
The detailed answer for the above question is provided belo...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


