Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Mass transfer operations SO2 is absorbed into water in a packed column at 323K and 101.3kPa. The equilibrium solubility of SO2 in water is given
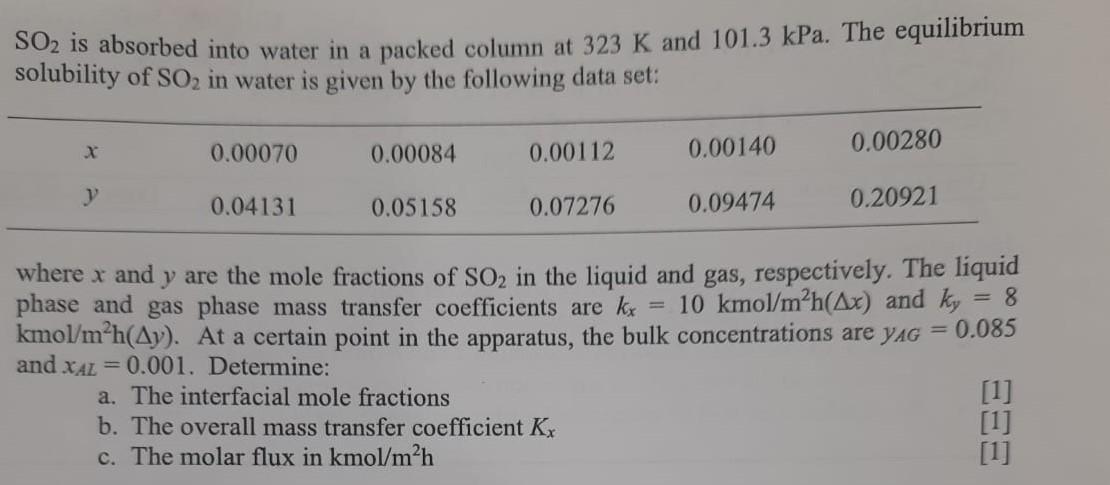 Mass transfer operations
Mass transfer operations
SO2 is absorbed into water in a packed column at 323K and 101.3kPa. The equilibrium solubility of SO2 in water is given by the following data set: where x and y are the mole fractions of SO2 in the liquid and gas, respectively. The liquid phase and gas phase mass transfer coefficients are kx=10kmol/m2h(x) and ky=8 kmol/m2h(y). At a certain point in the apparatus, the bulk concentrations are yAG=0.085 and xAL=0.001. Determine: a. The interfacial mole fractions b. The overall mass transfer coefficient Kx [1] c. The molar flux in kmol/m2h [1] [1]
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


