Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Match Each Item With The Correct Statement Below. Choose... Region Of High Probability Of Finding An Electron Tendency Of Electrons To Enter Orbitals Of Lowest
Match Each Item With The Correct Statement Below. Choose... Region Of High Probability Of Finding An Electron Tendency Of Electrons To Enter Orbitals Of Lowest Energy First Arrangement Of Electrons Around Atomic Nucleus Choose... Aufbau Principle Heisenberg Uncertainty Principle Electron Configuration Ground State Atomic Orbital Pauli Exclusion Principle
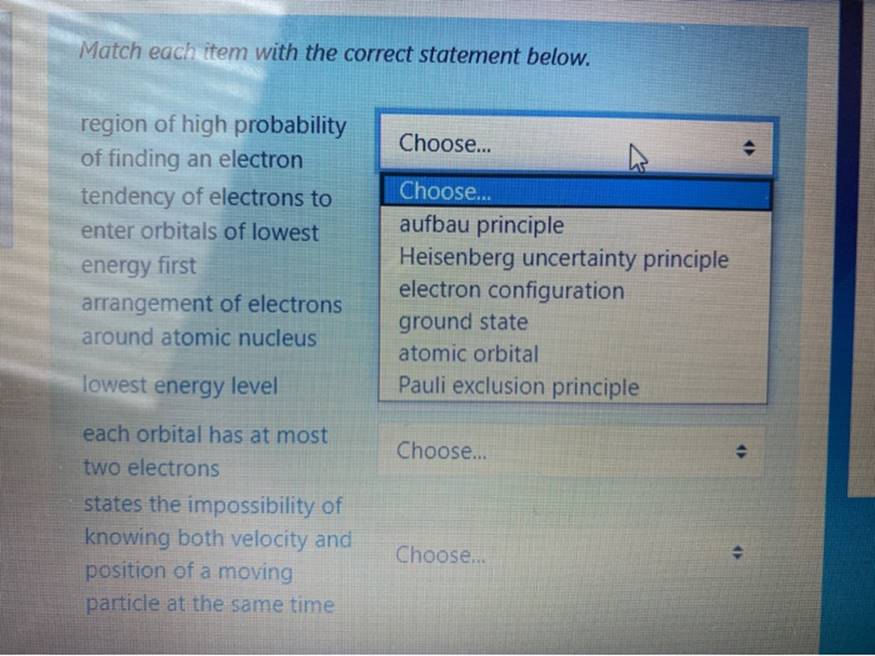
Match each item with the correct statement below. region of high probability of finding an electron tendency of electrons to enter orbitals of lowest energy first arrangement of electrons around atomic nucleus lowest energy level each orbital has at most two electrons states the impossibility of knowing both velocity and position of a moving Choose... Choose... aufbau principle Heisenberg uncertainty principle electron configuration ground state atomic orbital Pauli exclusion principle Choose... Choose... particle at the same time
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


