Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Match the rate expressions in LIST-I for the decomposition of X with the corresponding profiles provided in LIST-II. X, and k are constants having
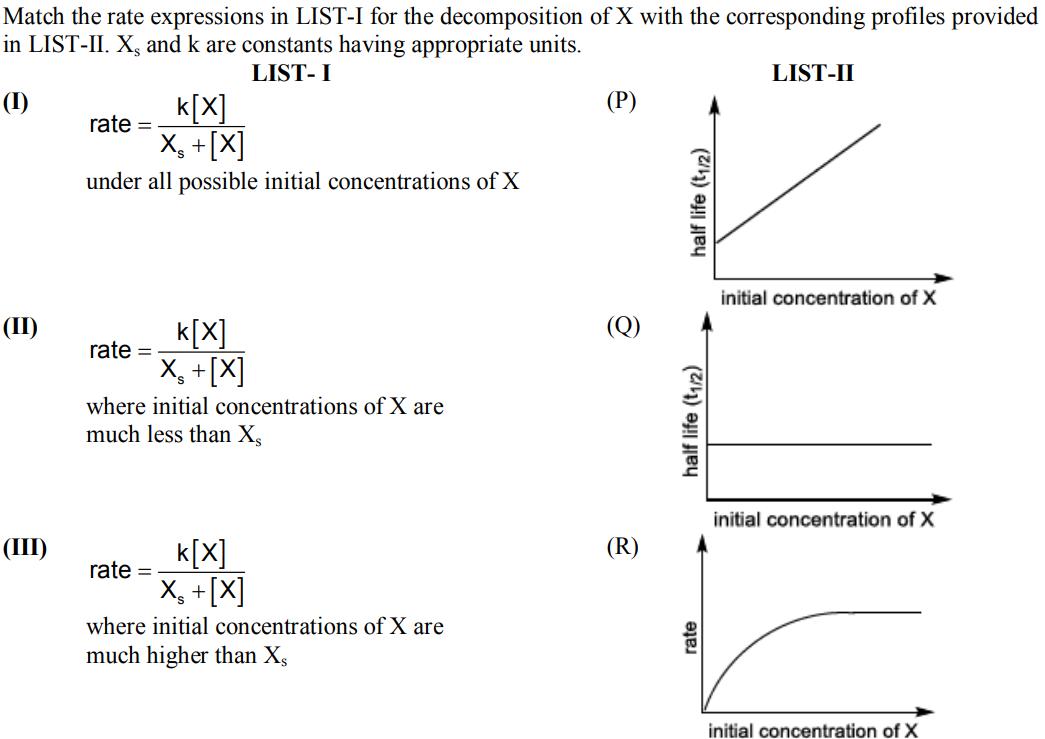

Match the rate expressions in LIST-I for the decomposition of X with the corresponding profiles provided in LIST-II. X, and k are constants having appropriate units. LIST-I (1) (II) (III) k[x] X +[X] under all possible initial concentrations of X rate = rate = k[X] X + [X] where initial concentrations of X are much less than X k[X] Xs +[X] where initial concentrations of X are much higher than X, rate = (P) (Q) (R) half life (1/2) half life (1/2) rate LIST-II initial concentration of X initial concentration of X initial concentration of X (IV) k[x] X +[X] where initial concentration of X is much higher than X, rate = (A) I P; IIQ; III S; IV T (C) I P; II Q; III Q; IV R (S) E [x]u[ [x] time time (B) I R; II S; III S; IV T (D) I R; II S; III Q; IV R
Step by Step Solution
★★★★★
3.34 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
The detailed answer for the a...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


