Question
Match the strongest expected IMF (dispersion, dipole, hydrogen bonding) to the following molecules. You may need to fill in any missing lone pair electrons!
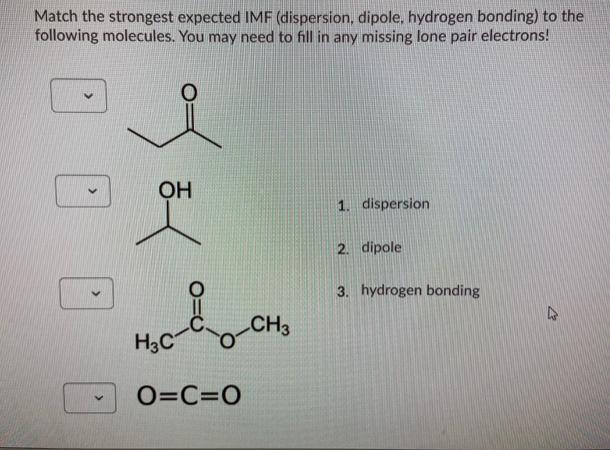
Match the strongest expected IMF (dispersion, dipole, hydrogen bonding) to the following molecules. You may need to fill in any missing lone pair electrons! OH ada H3C O=C=O CH3 1. dispersion 2. dipole 3. hydrogen bonding 2
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Because S DS Ketone of Polarity it has dipote d...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
College Physics
Authors: Raymond A. Serway, Jerry S. Faughn, Chris Vuille, Charles A. Bennett
7th Edition
9780534997236, 495113697, 534997236, 978-0495113690
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



