Answered step by step
Verified Expert Solution
Question
1 Approved Answer
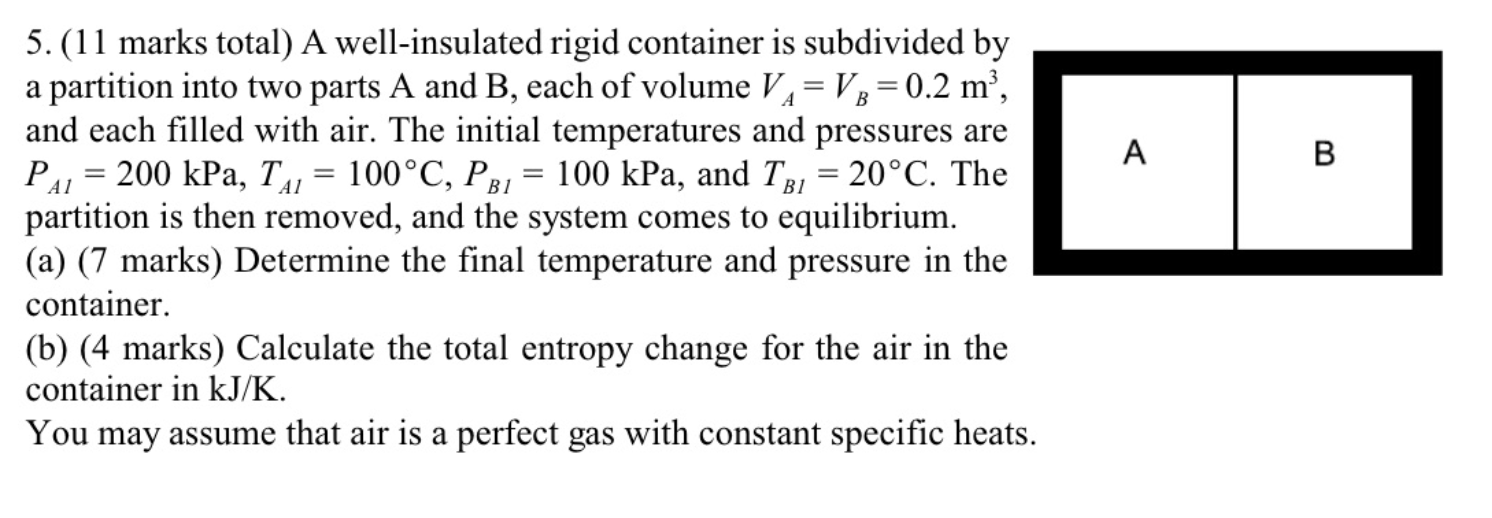
May need the formulas provided. Please give full solutions with steps. Will upvote 5. (11 marks total) A well-insulated rigid container is subdivided by a
May need the formulas provided. Please give full solutions with steps. Will upvote
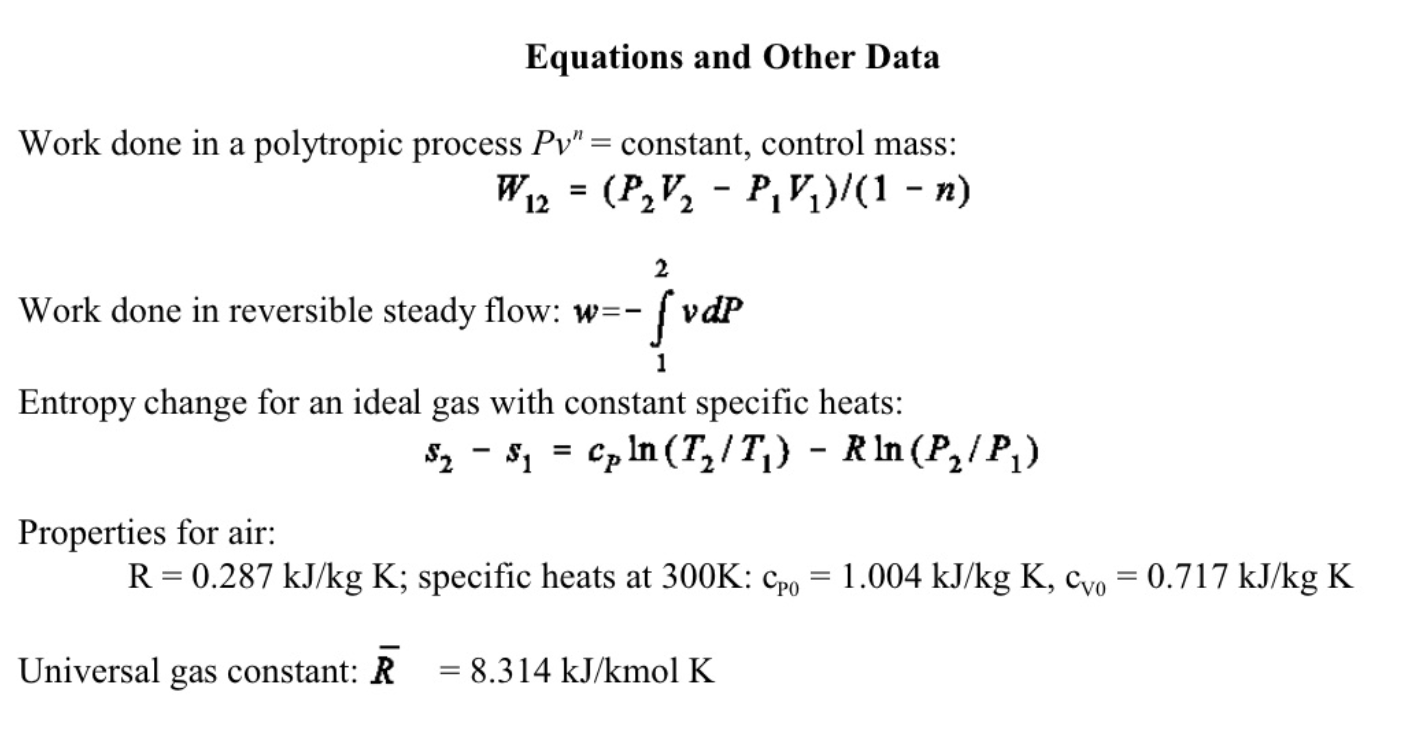
5. (11 marks total) A well-insulated rigid container is subdivided by a partition into two parts A and B, each of volume VA=VB=0.2m3, and each filled with air. The initial temperatures and pressures are PA1=200kPa,TA1=100C,PB1=100kPa, and TB1=20C. The partition is then removed, and the system comes to equilibrium. (a) (7 marks) Determine the final temperature and pressure in the container. (b) (4 marks) Calculate the total entropy change for the air in the container in kJ/K. You may assume that air is a perfect gas with constant specific heats Equations and Other Data Work done in a polytropic process Pvn= constant, control mass: W12=(P2V2P1V1)/(1n) Work done in reversible steady flow: w=12vdP Entropy change for an ideal gas with constant specific heats: s2s1=cPln(T2/T1)Rln(P2/P1) Properties for air: R=0.287kJ/kgK;specificheatsat300K:cP0=1.004kJ/kgK,cV0=0.717kJ/kgK Universal gas constant: R=8.314kJ/kmolKStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


