Question
Medical grade oxygen has become a product of national priority now. Oxygen concentration based on pressure swing adsorption is quickly becoming an obsolete technology. You
Medical grade oxygen has become a product of national priority now. Oxygen concentration based on pressure swing adsorption is quickly becoming an obsolete technology.
You are asked to dissect a paper and build a techno-economic study for a membrane based oxygen generation plant with 25 ton/day capacity and compare it with 1) cryogenic distillation and 2) pressure swing adsorption process.
For a given choice of polymer membrane, the techno-economic study should have the following components:
1) CAPEX
2) OPEX
3) List of major equipment
4) Pay back period
5) Reasons why membrane based process is being preferred over cryogenic distillation and PSA
Assume the plant has to be designed for a hospital in Punjab (ambient temperatures from 0 - 50 Deg C). The industrial electricity tariff is Rs 10 / kWh
6) How would opex change for this plant in Tamil Nadu?
7) Identify at least 20 different polymer membranes with different separation efficiencies and their corresponding prices. How would you choose a membrane for your plant?


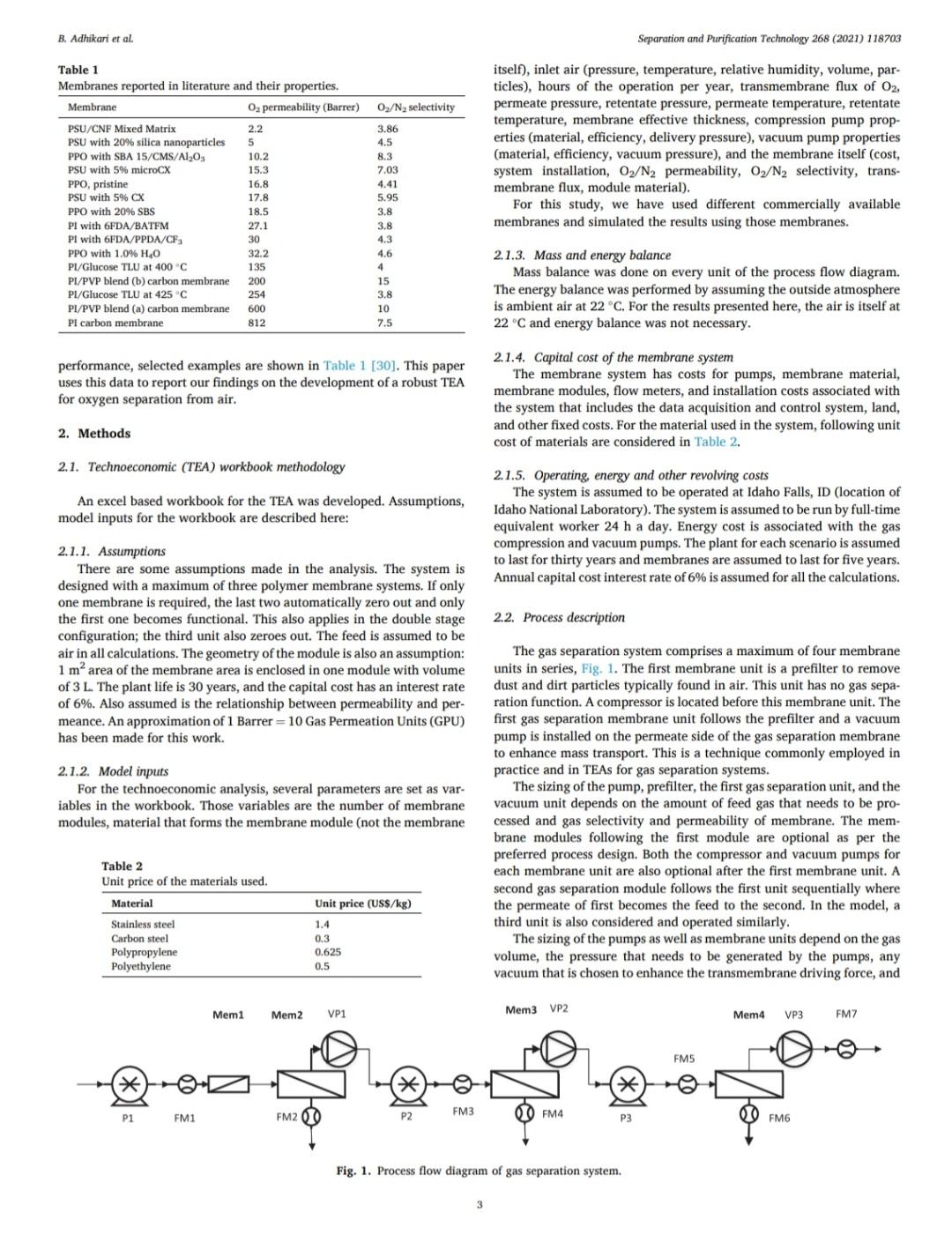
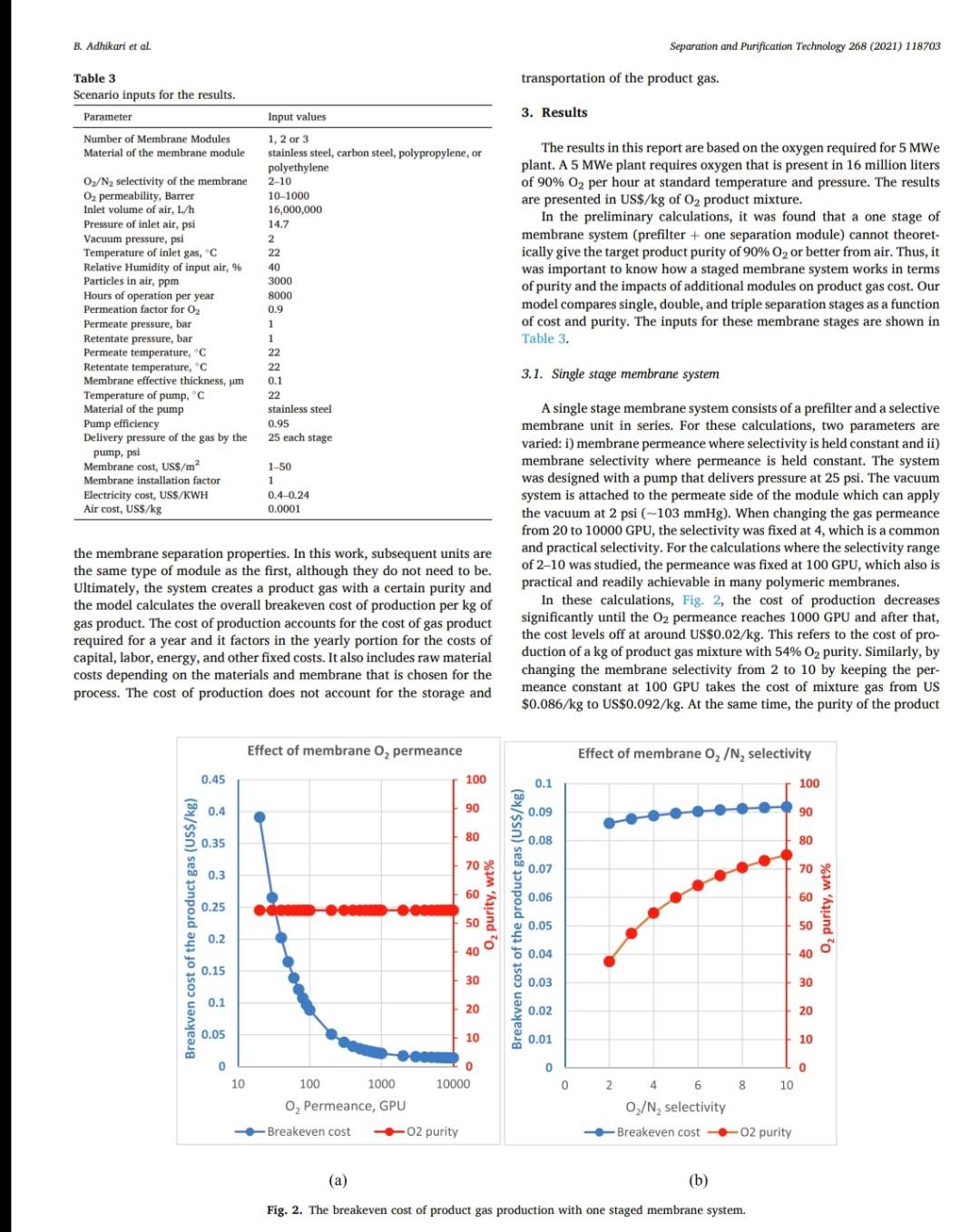
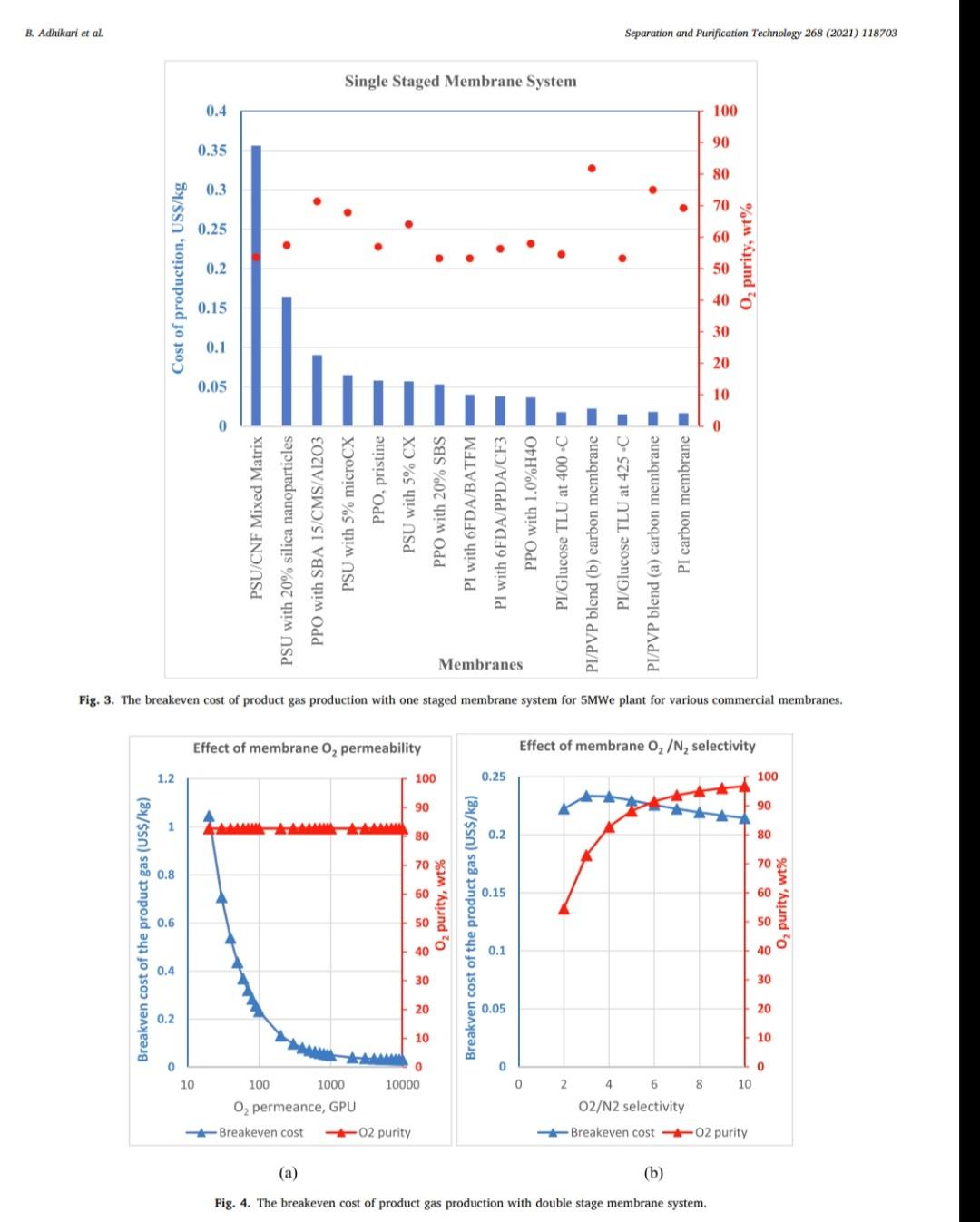
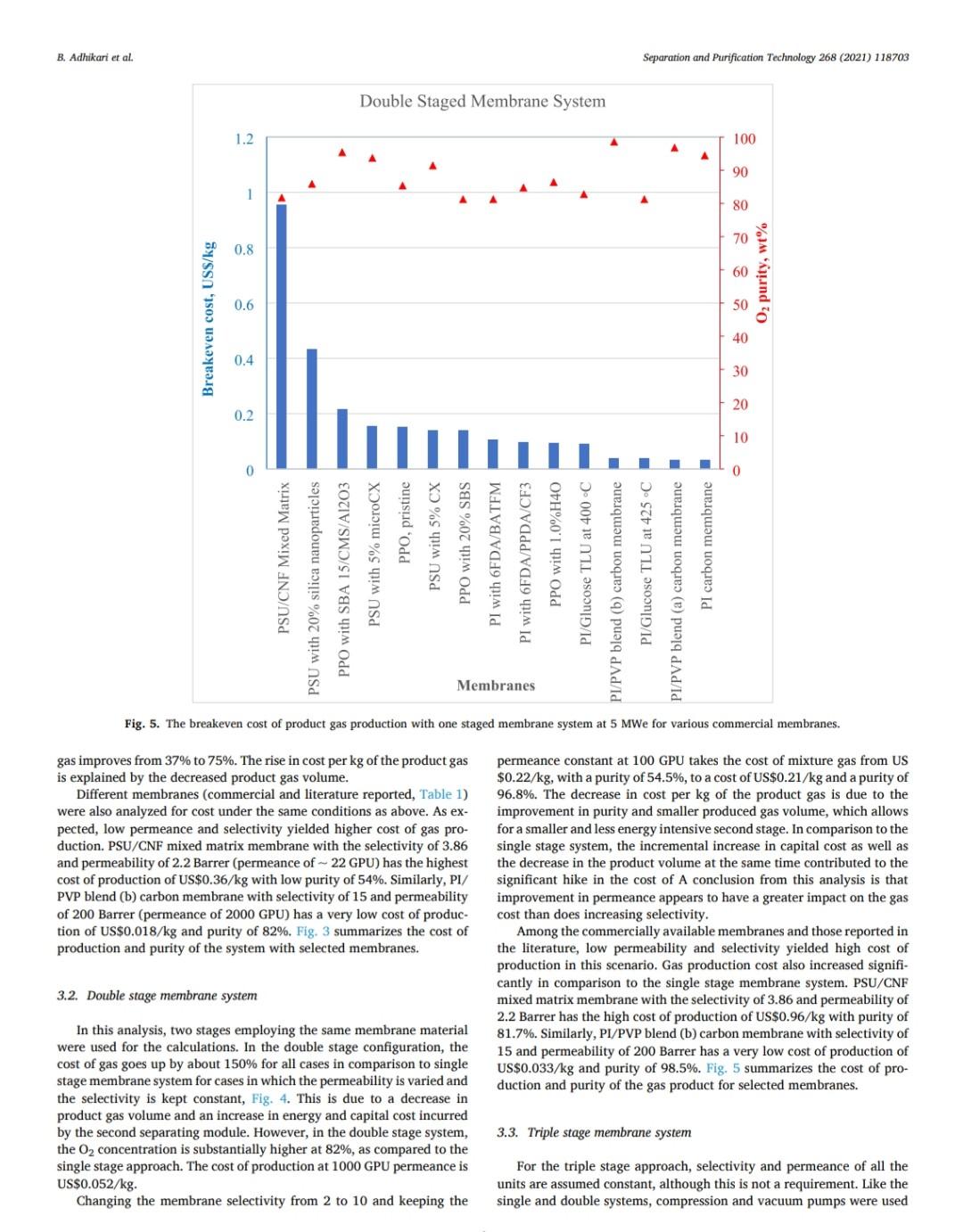
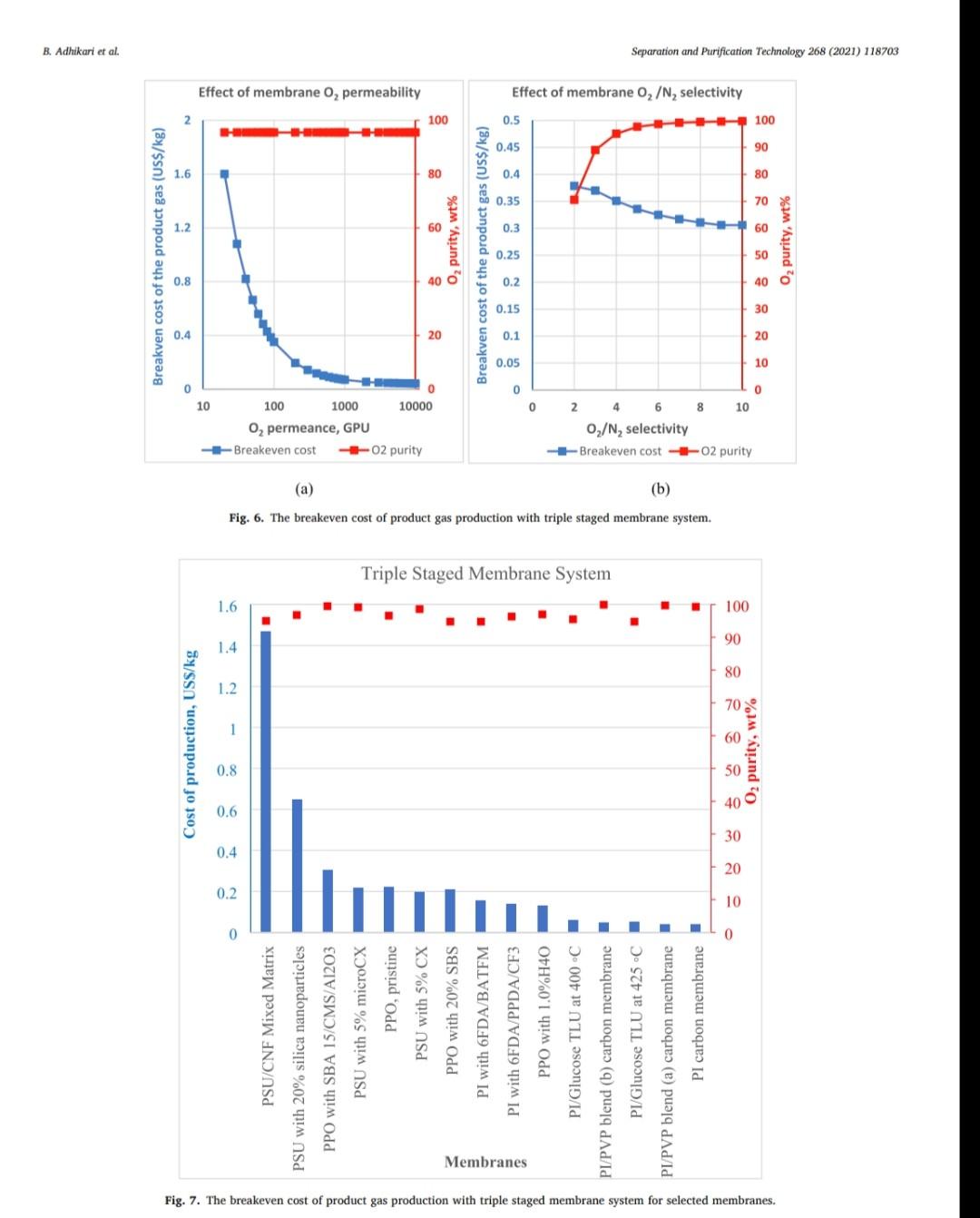
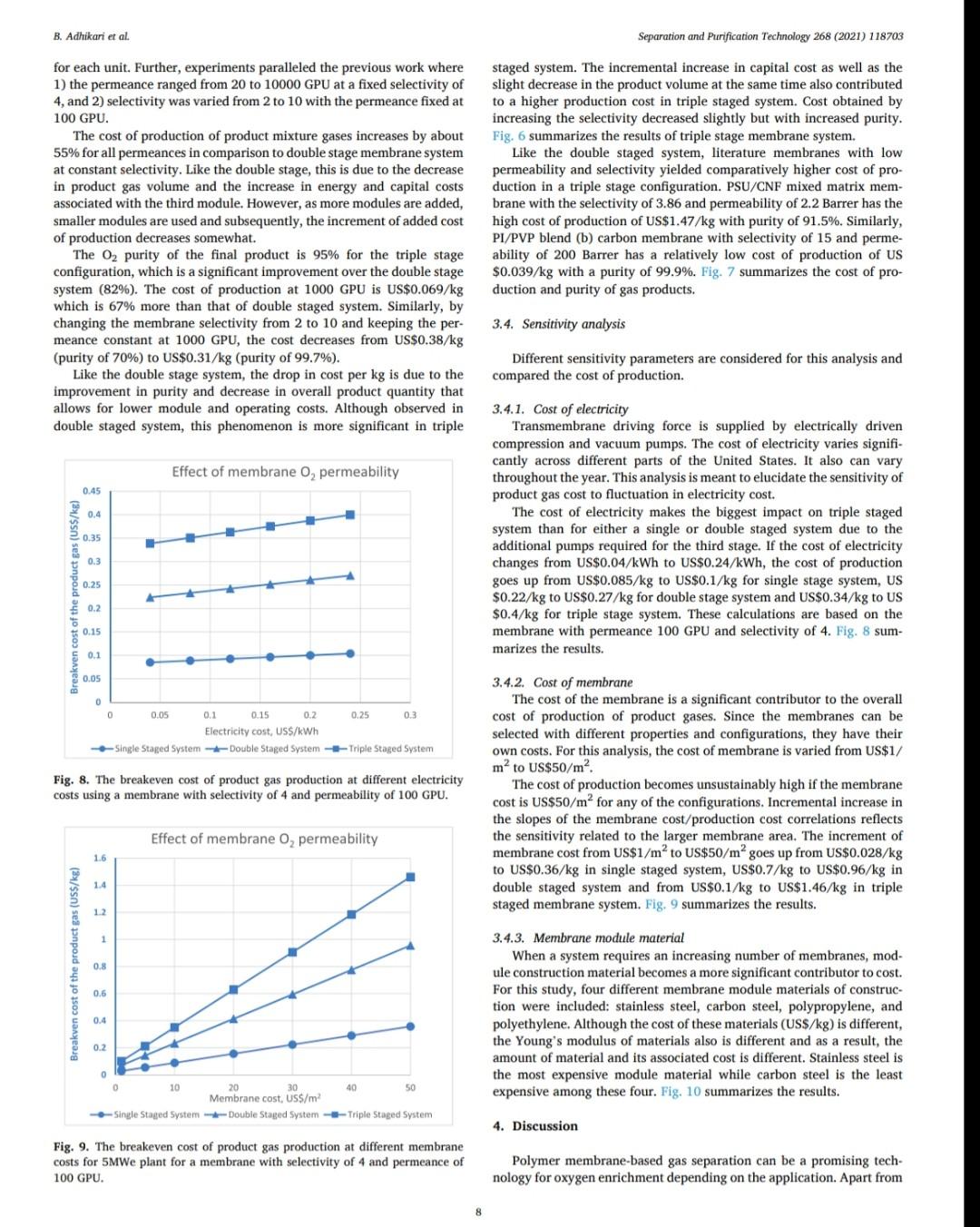
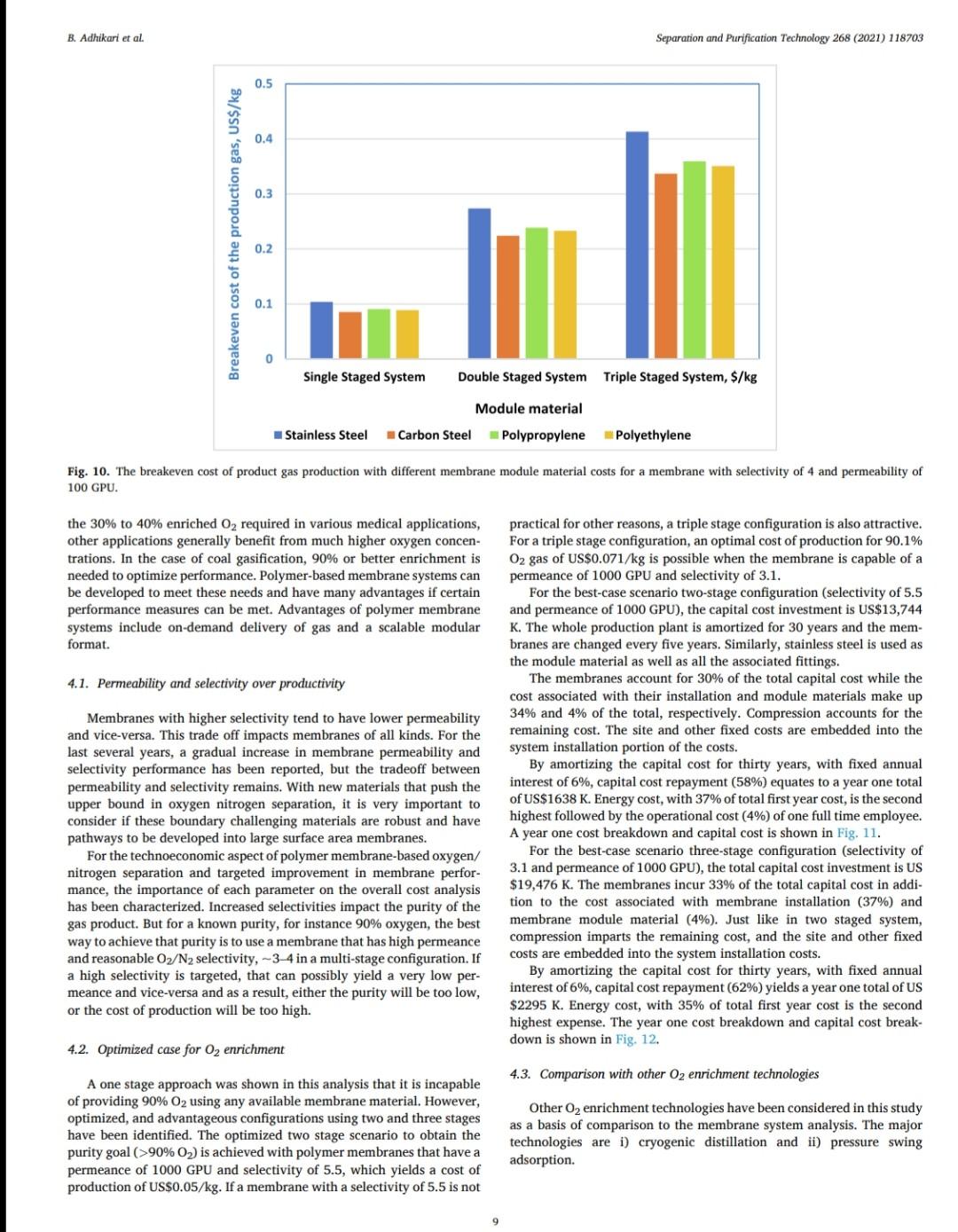
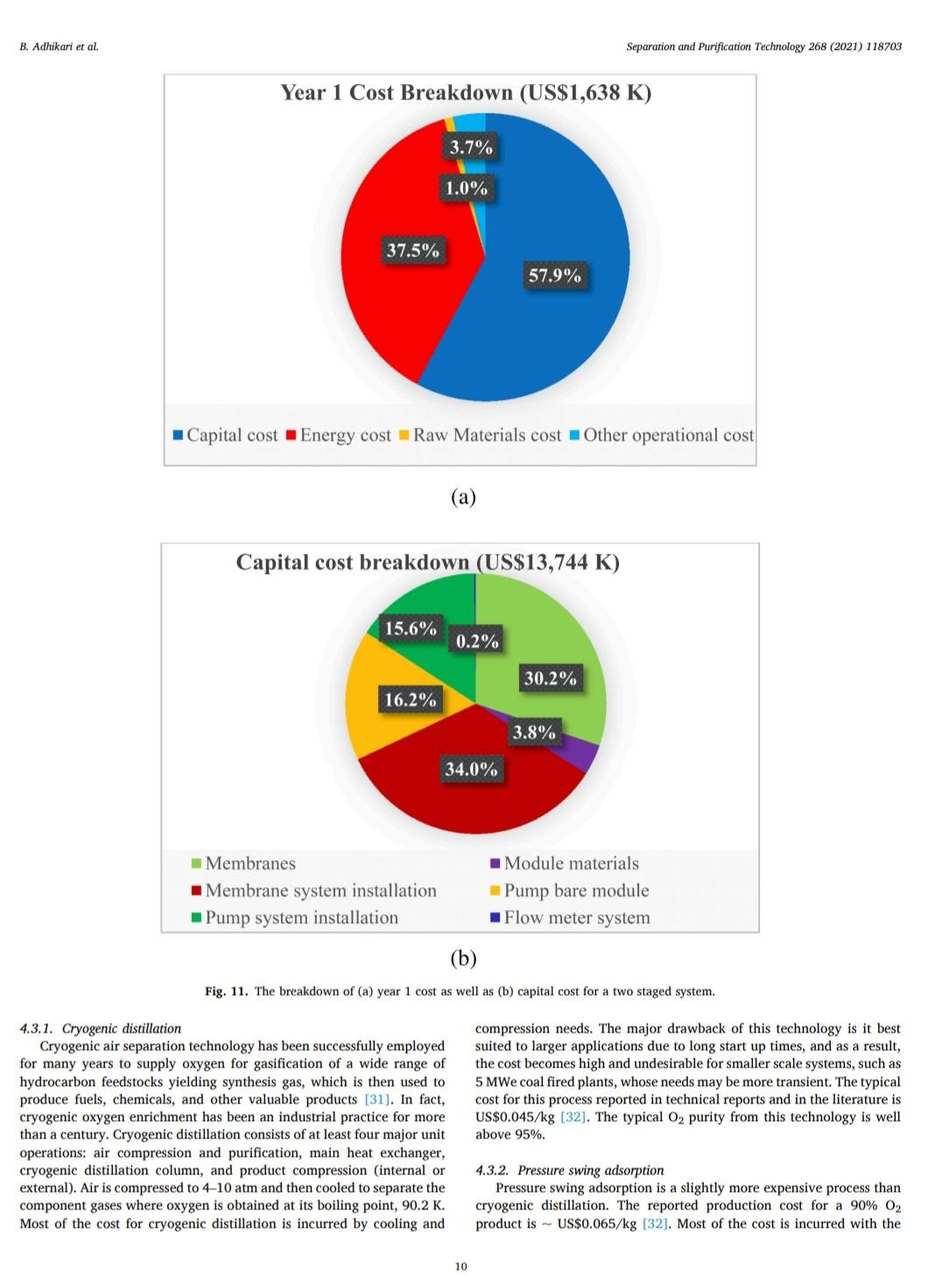
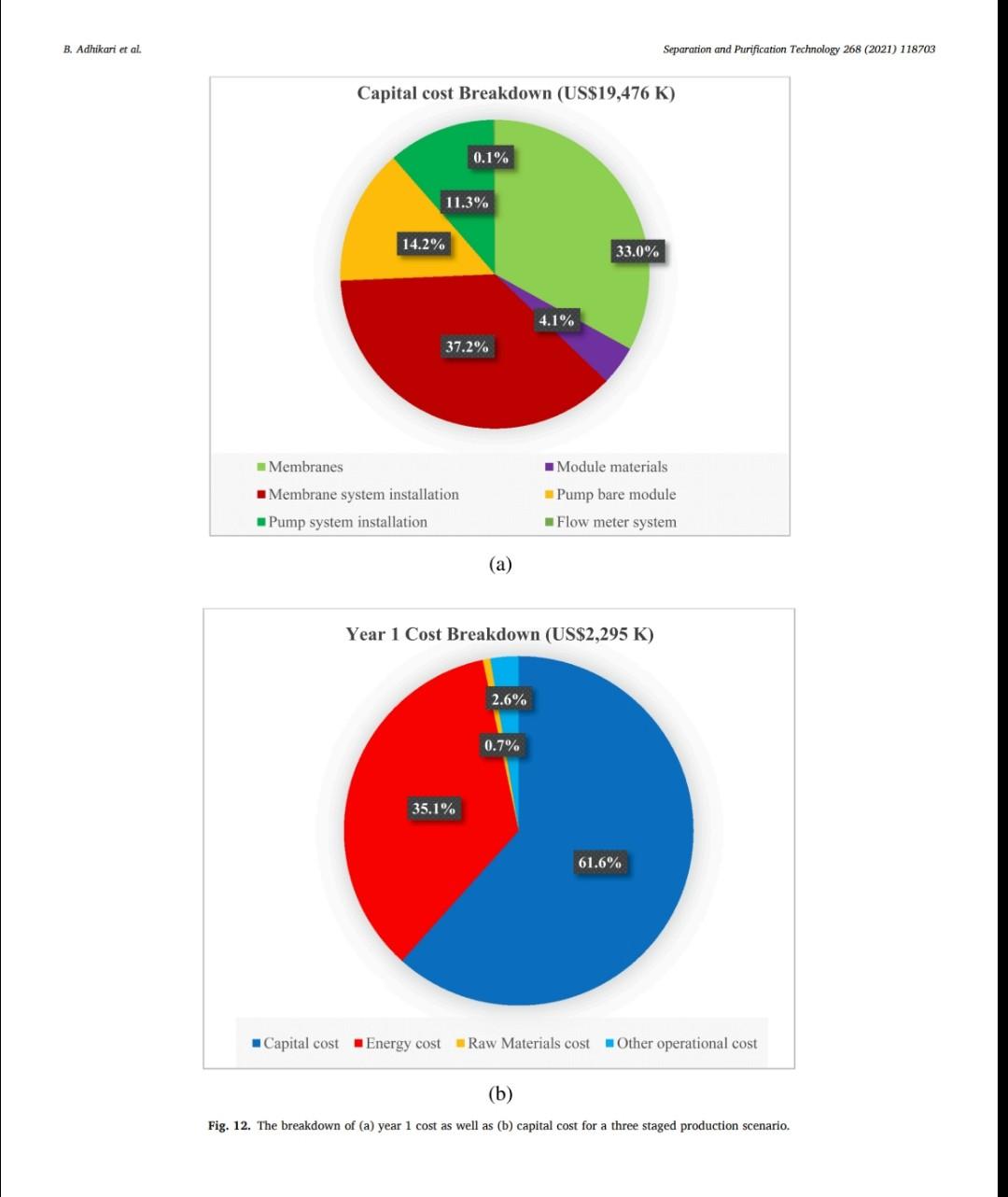

B. Adhikari et al. Separation and Purification Technology 268 (2021) 118703 depending on desired purity and production scale. For high purity gases, oxygen-nitrogen separation has been done using cryogenic distillation where the air is cooled down to a low temperature so that nitrogen condenses to liquid nitrogen. This process is known to produce high purity gas streams and is extensively used at large industrial scales (100-300 tons/day). However, this technology is known to be complex, expensive, and energy intensive [12]. The other major processes used are non-cryogenic methods such as pressure swing adsorption (PSA) and membrane separation methods. These methods are generally less energy intensive than cryogenic distillation and are best operated at mid-scale (20-100 tons/day). To support modular small scale (10-25 tons/day) deployment of coal-based power generation or gasification, flexible production processes that can quickly respond to rapid shifts in product demand based on membranes are gaining interest. To understand the overall economics of small-scale oxygen enrich- ment, we did comprehensive technoeconomic analyses (TEA) of different oxygen enrichment methods: cryogenic distillation, pressure swing adsorption, and membrane separation. The key performance measures in gas phase membrane separations are membrane perme- ability and selectivity. There is a well-known correlation in membrane performance called the "tradeoff', the higher the permeability the lower the selectivity and vice a versa (13). This study explores the impact of the tradeoff on oxygen production costs, as well as provides a basis of comparison between the three ap- proaches for use in small-scale deployments. This study reports the cost of oxygen production at different purity levels and under differing operating conditions where a desired purity level is achieved that fa- cilitates a comparison among differing membranes and production methods. Additionally, this study includes several sensitivity analyses to identify the most important contributors to production cost. have been investigated for various applications. Some adsorb nitrogen and others adsorb oxygen; therefore, the oxygen enrichment approaches can vary (18-20). The advantage of PSA over the cryogenic distillation is modularity better suited to small scale gas production with very low initial hold times. Similarly, PSA can be performed by using multiple beds in series [20]. As a result, the product purity easily can be manipulated based on the needs of the process and can be viable for small to mid-scale pro- duction; although it is better suited to mid-scale. In addition, PSA can be used to separate other gases as well [21,22). The disadvantage of PSA is very high capital cost as well as high operating costs. The modules are packed with expensive adsorbents, regenerated at high temperatures, that can rapidly degrade in terms of performance over time (water vapor adsorption). The smaller the system is, the higher the adsorption utili- zation limit and as a result, the performance of the system diminishes as it is reduced in size [23). 1.3. Membrane based processes 1.1. Cryogenic distillation Cryogenic distillation of air into its primary components (oxygen, nitrogen, argon, carbon dioxide and other gases) has been practiced for more than a century by taking advantage of the differences in the various gases boiling points. Cryogenic distillation is considered as a common route to produce high purity oxygen (above 99%) [10]. This technology uses air compressed to between 4 and 10 atm. The com- pressed air is then cooled to ambient temperature and passed through a pre-purification stage. Many of the trace components, namely water, carbon dioxide, and trace hydrocarbons, are eliminated at this point by passing the air through an adsorbent [14]. The purified air then enters the main cryogenic chamber where it is cooled to near its liquification temperature (approximately 100 K) before entering the distillation system. Oxygen is the highest boiling of all major components, so it is taken from the bottom of the low-pressure column, while nitrogen is taken from the top. Product streams are warmed to ambient temperature against incoming air (15). The main advantage of cryogenic distillation is its technological maturity and that it can separate large volumes of air with high purity. Also, the cost of production of oxygen and nitrogen are relatively low in comparison to other existing technologies. However, major drawbacks include a limited ability to economically produce smaller production volumes as well as high capital and energy costs [16]. Further, it re- quires significant time to come to equilibrium, so it is not well suited to intermittent operation. Membrane based technology for air separation is the most recently developed option among the three options. Using membranes, air can be separated into either component gases or enriched version of a component gas. Membrane processes are attractive alternatives to cryogenic distillation and PSA because they do not require regenerative steps and the gas products can be directly used or discharged [24). In addition, membranes have very little equilibration time requirement that lowers energy intensity (25,26). The membrane-based gas permeation process has gained interest over cryogenic distillation and PSA due to compact size, modular con- figurations, lower specific power consumption, reduced footprint, and facile approaches to small scale gas production at manageable cost. Several options exist with how membranes can be used depending upon the application, which gives flexibility to process design. In general, a better performing membrane helps save capital and energy costs. The field of membrane science is constantly evolving to create better and optimized membrane materials with higher selectivity and higher permeability [24,27). Polymeric membranes are sometimes preferred over ceramic and metallic membranes because they are relatively easy to form into large scale modules, can be mass produced, and can have minimal environ- mental impact. Although polymeric membran are prone to fouling in some applications, they have significantly reduced capital costs in comparison to other membranes and they can easily be modified and fabricated according to the specified requirements [28]. Furthermore, polymeric membranes have competitive selectivity and permeability with ceramic and metallic counterparts [28]. They also can be easier to handle because they are not as brittle as metal or ceramic materials, and high-surface area membrane modules can easily be built with a rela- tively small footprint. Polymeric membranes can be used to form large surface area hollow fiber membranes with a relatively small footprint at reasonably low cost of production [29]. Oxygen and nitrogen separation from air is important due to the demand for these gases, especially the need to deliver them at low cost while generating them in a range spanning modular portable units (assisted oxygen) up to industrial scale. Because of the limitations of cryogenics and PSA in terms of cost and sizing, membrane technologies are gaining ground specifically in smaller scale production scenarios. Membrane-based separation is gaining traction over the last two decades due to several advancements that improve the selectivity and the permeate flux at ambient operating conditions. As a result, the pro- duction costs for gaseous products are falling, and not just for air sep- arations but in other gas separation applications as well. Although membrane processes have been demonstrated to produce oxygen as pure as 90%, the focus has been on needs for oxygen in the 30-40% (medical applications) range, while also requiring low production cost [24]. The literature is replete with examples of 02/N2 separation 1.2. Pressure swing adsorption Pressure swing adsorption (PSA) has been used in the oxygen market for last several decades producing high purity oxygen. In this technol. ogy, a packed column with adsorbents, such as a zeolite, adsorbs a component, typically nitrogen, at one pressure and releases that same component at another pressure [17]. Different adsorbents such as car- bon molecular sieve, zeolite 5A, zeolite 13X, polyethyleneimine, etc. B. Adhikari et al. Separation and Purification Technology 268 (2021) 118703 transportation of the product gas. Table 3 Scenario inputs for the results. Parameter Number of Membrane Modules Material of the membrane module 3. Results Input values 1, 2 or 3 stainless steel, carbon steel, polypropylene, or polyethylene 2-10 10-1000 16,000,000 14.2 The results in this report are based on the oxygen required for 5 MWe plant. A 5 MWe plant requires oxygen that is present in 16 million liters of 90% O2 per hour at standard temperature and pressure. The results are presented in US$/kg of O2 product mixture. In the preliminary calculations, it was found that a one stage of membrane system (prefilter + one separation module) cannot theoret- ically give the target product purity of 90% O, or better from air. Thus, it was important to know how a staged membrane system works in terms of purity and the impacts of additional modules on product gas cost. Our model compares single, double, and triple separation stages as a function of cost and purity. The inputs for these membrane stages are shown in Table 3. 02/N, selectivity of the membrane O2 permeability, Barrer Inlet volume of air, L/h Pressure of inlet air, psi Vacuum pressure, psi Temperature of inlet gas, C Relative Humidity of input air, % Particles in air, ppm Hours of operation per year Permeation factor for Oz Fermeate presse Permeate pressure, bar Permeate temperature, Retentate temperature, C Mahoff Membrane effective thickness, um Temperature of pump. C Material of the pump Pump efficiency Delivery pressure of the gas by the pump, psi Membrane cost, USS/m2 Membrane installation factor Electricity cost, USS/KWH Air cost, USS/kg 22 40 3000 8000 0.9 Ketentate pressure, bar 3.1. Single stage membrane system 22 22 0.1 22 stainless steel 0.95 25 each stage 1-50 1 0.4-0.24 0.0001 the membrane separation properties. In this work, subsequent units are the same type of module as the first, although they do not need to be. Ultimately, the system creates a product gas with a certain purity and the model calculates the overall breakeven cost of production per kg of gas product. The cost of production accounts for the cost of gas product required for a year and it factors in the yearly portion for the costs of capital, labor, energy, and other fixed costs. It also includes raw material costs depending on the materials and membrane that is chosen for the process. The cost of production does not account for the storage and A single stage membrane system consists of a prefilter and a selective membrane unit in series. For these calculations, two parameters are varied: i) membrane permeance where selectivity is held constant and ii) membrane selectivity where permeance is held constant. The system was designed with a pump that delivers pressure at 25 psi. The vacuum system is attached to the permeate side of the module which can apply the vacuum at 2 psi (~103 mmHg). When changing the gas permeance from 20 to 10000 GPU, the selectivity was fixed at 4, which is a common and practical selectivity. For the calculations where the selectivity range of 2-10 was studied, the permeance was fixed at 100 GPU, which also is practical and readily achievable in many polymeric membranes. In these calculations, Fig. 2, the cost of production decreases significantly until the O2 permeance reaches 1000 GPU and after that, the cost levels off at around US$0.02/kg. This refers to the cost of pro- duction of a kg of product gas mixture with 54% O2 purity. Similarly, by changing the membrane selectivity from 2 to 10 by keeping the per- meance constant at 100 GPU takes the cost of mixture gas from US $0.086/kg to US$0.092/kg. At the same time, the purity of the product Effect of membrane Opermeance Effect of membrane 02/N, selectivity 0.45 100 0.1 100 0.4 90 0.09 90 80 0.35 0.08 80 70 0.07 70 0.3 60 0.06 60 0.25 Breakven cost of the product gas (US$/kg) 50 O2 purity, wt% Breakven cost of the product gas (US$/kg) Ozpurity, wt% 0.05 50 0.2 40 6 0.04 40 0.15 30 8 0.03 30 0.1 20 0.02 20 0.05 10 10 0 0.01 0 0 0 10000 10 100 1000 0 O, Permeance, GPU 0 2 4 6 8 10 Oz/N, selectivity Breakeven cost -02 purity -Breakeven cost -02 purity (a) (b) Fig. 2. The breakeven cost of product gas production with one staged membrane system. B. Adhikari et al. Breakven cost of the product gas (US$/kg) 0.1 0.4 1.2 1 Cost of production, US$/kg PSU/CNF Mixed Matrix PSU with 20% silica nanoparticles . Breakeven cost 100 O, permeance, GPU 1000 02 purity 10000 Effect of membrane 0, permeability PPO with SBA 15/CMS/A1203 PSU with 5% microCX PPO, pristine PSU with 5% CX 100 O2 purity, wt% PPO with 20% SBS (a) Fig. 4. The breakeven cost of product gas production with double stage membrane system. (b) Breakven cost of the product gas (US$/kg) Fig. 3. The breakeven cost of product gas production with one staged membrane system for 5MWe plant for various commercial membranes. PI with 6FDA/BATEM Membranes Single Staged Membrane System 0.05 0.15 0.2 0.25 0 PI with 6FDA/PPDA/CF3 0 PPO with 1.0%H40 PI/Glucose TLU at 400C 2. PI/PVP blend (b) carbon membrane 4 02/N2 selectivity PI/Glucose TLU at 425 C 1 Breakeven cost-02 purity Effect of membrane 02/N, selectivity PI/PVP blend (a) carbon membrane PI carbon membrane 8 10 O, purity, wt% Separation and Purification Technology 268 (2021) 118703 O2 purity, wt% B. Adhikari et al. Separation and Purification Technology 268 (2021) 118703 Double Staged Membrane System 1.2. 100 90 1 80 70 0.8 60 0.6 O2 purity, wt% 50 Breakeven cost, USS/kg 40 0.4 30 20 0.2 10 0 PSU/CNF Mixed Matrix PSU with 20% silica nanoparticles PPO with SBA 15/CMS/A1203 PPO, pristine PSU with 5% microCX PSU with 5% CX PPO with 20% SBS PI with 6FDA/BATFM PI with 6FDA/PPDA/CF3 PPO with 1.0%H40 PI/Glucose TLU at 400C - PI/PVP blend (b) carbon membrane - PI/Glucose TLU at 425 C PI/PVP blend (a) carbon membrane PI carbon membrane Membranes Fig. 5. The breakeven cost of product gas production with one staged membrane system at 5 MWe for various commercial membranes. gas improves from 37% to 75%. The rise in cost per kg of the product gas is explained by the decreased product gas volume. Different membranes (commercial and literature reported, Table 1) were also analyzed for cost under the same conditions as above. As ex- pected, low permeance and selectivity yielded higher cost of gas pro- duction. PSU/CNF mixed matrix membrane with the selectivity of 3.86 and permeability of 2.2 Barrer (permeance of -22 GPU) has the highest cost of production of US$0.36/kg with low purity of 54%. Similarly, PI/ PVP blend (b) carbon membrane with selectivity of 15 and permeability of 200 Barrer (permeance of 2000 GPU) has a very low cost of produc- tion of US$0.018/kg and purity of 82%. Fig. 3 summarizes the cost of production and purity of the system with selected membranes. permeance constant at 100 GPU takes the cost of mixture gas from US $0.22/kg, with a purity of 54.5%, to a cost of US$0.21/kg and a purity of 96.8%. The decrease in cost per kg of the product gas is due to the improvement in purity and smaller produced gas volume, which allows for a smaller and less energy intensive second stage. In comparison to the single stage system, the incremental increase in capital cost as well as the decrease in the product volume at the same time contributed to the significant hike in the cost of A conclusion from this analysis is that improvement in permeance appears to have a greater impact on the gas cost than does increasing selectivity. Among the commercially available membranes and those reported in the literature, low permeability and selectivity yielded high cost of production in this scenario. Gas production cost also increased signifi- cantly in comparison to the single stage membrane system. PSU/CNF mixed matrix membrane with the selectivity of 3.86 and permeability of 2.2 Barrer has the high cost of production of US$0.96/kg with purity of 81.7%. Similarly, PI/PVP blend (b) carbon membrane with selectivity of 15 and permeability of 200 Barrer has a very low cost of production of US$0.033/kg and purity of 98.5%. Fig. 5 summarizes the cost of pro- duction and purity of the gas product for selected membranes. 3.2. Double stage membrane system In this analysis, two stages employing the same membrane material were used for the calculations. In the double stage configuration, the cost of gas goes up by about 150% for all cases in comparison to single stage membrane system for cases in which the permeability is varied and the selectivity is kept constant, Fig. 4. This is due to a decrease in product gas volume and an increase in energy and capital cost incurred by the second separating module. However, in the double stage system, the O2 concentration is substantially higher at 82%, as compared to the single stage approach. The cost of production at 1000 GPU permeance is US$0.052/kg. Changing the membrane selectivity from 2 to 10 and keeping the 3.3. Triple stage membrane system For the triple stage approach, selectivity and permeance of all the units are assumed constant, although this is not a requirement. Like the single and double systems, compression and vacuum pumps were used B. Adhikari et al. Breakven cost of the product gas (US$/kg) 0.4 0.8 Cost of production, USS/kg 1.2 1.6 TO 0.4 1.4 1.6 0 1 PSU/CNF Mixed Matrix PSU with 20% silica nanoparticles (a) Breakeven cost 100 02 permeance, GPU 1000 -H -02 purity 10000 Effect of membrane 0, permeability PPO with SBA 15/CMS/A1203 PSU with 5% microCX PPO, pristine PSU with 5% CX 20 80 100 PPO with 20% SBS Fig. 7. The breakeven cost of product gas production with triple staged membrane system for selected membranes. Membranes Fig. 6. The breakeven cost of product gas production with triple staged membrane system. Triple Staged Membrane System O, purity, wt% Breakven cost of the product gas (US$/kg) PI with 6FDA/BATFM PI with 6FDA/PPDA/CF3 S0 0.1 0.3 0.25 0.5 . 0 PPO with 1.0%H40 PI/Glucose TLU at 400 C PI/PVP blend (b) carbon membrane PI/Glucose TLU at 425 C Effect of membrane o,/N, selectivity PI/PVP blend (a) carbon membrane ! (b) 9 2 4 Breakeven cost-02 purity 0,/N, selectivity 8. 10 Pl carbon membrane 1 O2 purity, wt% 100 Separation and Purification Technology 268 (2021) 118703 O2 purity, wt% B. Adhikari et al. Separation and Purification Technology 268 (2021) 118703 for each unit. Further, experiments paralleled the previous work where 1) the permeance ranged from 20 to 10000 GPU at a fixed selectivity of 4, and 2) selectivity was varied from 2 to 10 with the permeance fixed at 100 GPU. The cost of production of product mixture gases increases by about 55% for all permeances in comparison to double stage membrane system at constant selectivity. Like the double stage, this is due to the decrease in product gas volume and the increase in energy and capital costs associated with the third module. However, as more modules are added, smaller modules are used and subsequently, the increment of added cost of production decreases somewhat. The O2 purity of the final product is 95% for the triple stage configuration, which is a significant improvement over the double stage system (82%). The cost of production at 1000 GPU is US$0.069/kg which is 67% more than that of double staged system. Similarly, by changing the membrane selectivity from 2 to 10 and keeping the per- meance constant at 1000 GPU, the cost decreases from US$0.38/kg (purity of 70%) to US$0.31/kg (purity of 99.7%). Like the double stage system, the drop in cost per kg is due to the improvement in purity and decrease in overall product quantity that allows for lower module and operating costs. Although observed in double staged system, this phenomenon is more significant in triple staged system. The incremental increase in capital cost as well as the slight decrease in the product volume at the same time also contributed to a higher production cost in triple staged system. Cost obtained by increasing the selectivity decreased slightly but with increased purity. Fig. 6 summarizes the results of triple stage membrane system. Like the double staged system, literature membranes with low permeability and selectivity yielded comparatively higher cost of pro- duction in a triple stage configuration. PSU/CNF mixed matrix mem- brane with the selectivity of 3.86 and permeability of 2.2 Barrer has the high cost of production of US$1.47/kg with purity of 91.5%. Similarly, PI/PVP blend (b) carbon membrane with selectivity of 15 and perme- ability of 200 Barrer has a relatively low cost of production of US $0.039/kg with a purity of 99.9%. Fig. 7 summarizes the cost of pro- duction and purity of gas products. 3.4. Sensitivity analysis Different sensitivity parameters are considered for this analysis and compared the cost of production. Effect of membrane o, permeability 0.45 0.4 3.4.1. Cost of electricity Transmembrane driving force is supplied by electrically driven compression and vacuum pumps. The cost of electricity varies signifi- cantly across different parts of the United States. It also can vary throughout the year. This analysis is meant to elucidate the sensitivity of product gas cost to fluctuation in electricity cost. The cost of electricity makes the biggest impact on triple staged system than for either a single or double staged system due to the additional pumps required for the third stage. If the cost of electricity changes from US$0.04/kWh to US$0.24/kWh, the cost of production goes up from US$0.085/kg to US$0.1/kg for single stage system, US $0.22/kg to US$0.27/kg for double stage system and US$0.34/kg to US $0.4/kg for triple stage system. These calculations are based on the membrane with permeance 100 GPU and selectivity of 4. Fig. 8 sum- marizes the results. 0.35 0.3 0.25 Breakven cost of the product gas (US$/kg) $ 0.2 0.15 0.1 . . 0.05 0 0 0.05 0.1 0.15 0.2 0.25 03 Electricity cost, USS/kWh Single Staged System Double Staged System Triple Staged System Fig. 8. The breakeven cost of product gas production at different electricity costs using a membrane with selectivity of 4 and permeability of 100 GPU. 3.4.2. Cost of membrane The cost of the membrane is a significant contributor to the overall cost of production of product gases. Since the membranes can be selected with different properties and configurations, they have their own costs. For this analysis, the cost of membrane is varied from US$1/ m to US$50/m The cost of production becomes unsustainably high if the membrane cost is US$50/m for any of the configurations. Incremental increase in the slopes of the membrane cost/production cost correlations reflects the sensitivity related to the larger membrane area. The increment of membrane cost from US$1/m to US$50/m goes up from US$0.028/kg to US$0.36/kg in single staged system, US$0.7/kg to US$0.96/kg in double staged system and from US$0.1/kg to US$1.46/kg in triple staged membrane system. Fig. 9 summarizes the results. Effect of membrane o, permeability 1.6 1.4 12 1 Breakven cost of the product gas (US$/kg) 0.8 0.6 3.4.3. Membrane module material When a system requires an increasing number of membranes, mod- ule construction material becomes a more significant contributor to cost. For this study, four different membrane module materials of construc- tion were included: stainless steel, carbon steel, polypropylene, and polyethylene. Although the cost of these materials (US$/kg) is different, the Young's modulus of materials also is different and as a result, the amount of material and its associated cost is different. Stainless steel is the most expensive module material while carbon steel is the least expensive among these four. Fig. 10 summarizes the results. 0.4 0.2 0 0 10 20 30 40 50 Membrane cost, US$/m Single Staged System Double Staged System --Triple Staged System 4. Discussion Fig. 9. The breakeven cost of product gas production at different membrane costs for 5MWe plant for a membrane with selectivity of 4 and permeance of 100 GPU. Polymer membrane-based gas separation can be a promising tech- nology for oxygen enrichment depending on the application. Apart from 8 B. Adhikari et al. Separation and Purification Technology 268 (2021) 118703 0.5 0.4 0.3 Breakeven cost of the production gas, US$/kg 0.2 0.1 0 Single Staged System Double Staged System Triple Staged System, $/kg Module material Stainless Steel Carbon Steel Polypropylene Polyethylene Fig. 10. The breakeven cost of product gas production with different membrane module material costs for a membrane with selectivity of 4 and permeability of 100 GPU. the 30% to 40% enriched O2 required in various medical applications, other applications generally benefit from much higher oxygen concen- trations. In the case of coal gasification, 90% or better enrichment is needed to optimize performance. Polymer-based membrane systems can be developed to meet these needs and have many advantages if certain performance measures can be met. Advantages of polymer membrane systems include on-demand delivery of gas and a scalable modular format. 4.1. Permeability and selectivity over productivity Membranes with higher selectivity tend to have lower permeability and vice-versa. This trade off impacts membranes of all kinds. For the last several years, a gradual increase in membrane permeability and selectivity performance has been reported, but the tradeoff between permeability and selectivity remains. With new materials that push the upper bound in oxygen nitrogen separation, it is very important to consider if these boundary challenging materials are robust and have pathways to be developed into large surface area membranes. For the technoeconomic aspect of polymer membrane-based oxygen/ nitrogen separation and targeted improvement in membrane perfor- mance, the importance of each parameter on the overall cost analysis has been characterized. Increased selectivities impact the purity of the gas product. But for a known purity, for instance 90% oxygen, the best way to achieve that purity is to use a membrane that has high permeance and reasonable Oz/N2 selectivity, -3-4 in a multi-stage configuration. If a high selectivity is targeted, that can possibly yield a very low per- meance and vice-versa and as a result, either the purity will be too low, or the cost of production will be too high. practical for other reasons, a triple stage configuration is also attractive. For a triple stage configuration, an optimal cost of production for 90.1% O2 gas of US$0.071/kg is possible when the membrane is capable of a permeance of 1000 GPU and selectivity of 3.1, For the best-case scenario two-stage configuration (selectivity of 5.5 and permeance of 1000 GPU), the capital cost investment is US$13,744 K. The whole production plant is amortized 30 years and the mem- branes are changed every five years. Similarly, stainless steel is used as the module material as well as all the associated fittings. The membranes account for 30% of the total capital cost while the cost associated with their installation and module materials make up 34% and 4% of the total, respectively. Compression accounts for the remaining cost. The site and other fixed costs are embedded into the system installation portion of the costs. By amortizing the capital cost for thirty years, with fixed annual interest of 6%, capital cost repayment (58%) equates to a year one total of US$1638 K. Energy cost, with 37% of total first year cost, is the second highest followed by the operational cost (4%) of one full time employee. A year one cost breakdown and capital cost is shown in Fig. 11. For the best-case scenario three-stage configuration (selectivity of 3.1 and permeance of 1000 GPU), the total capital cost investment is US $19,476 K. The membranes incur 33% of the total capital cost in addi- tion to the cost associated with membrane installation (37%) and membrane module material (4%). Just like in two staged system, compression imparts the remaining cost, and the site and other fixed costs are embedded into the system installation costs. By amortizing the capital cost for thirty years, with fixed annual interest of 6%, capital cost repayment (62%) yields a year one total of US $2295 K. Energy cost, with 35% of total first year cost is the second highest expense. The year one cost breakdown and capital cost break- down is shown in Fig. 12. 4.2. Optimized case for O2 enrichment 4.3. Comparison with other O2 enrichment technologies A one stage approach was shown in this analysis that it is incapable of providing 90% O2 using any available membrane material. However, optimized, and advantageous configurations using two and three stages have been identified. The optimized two stage scenario to obtain the purity goal (>90% O2) is achieved with polymer membranes that have a permeance of 1000 GPU and selectivity of 5.5, which yields a cost of production of US$0.05/kg. If a membrane with a selectivity of 5.5 is not Other Oz enrichment technologies have been considered in this study as a basis of comparison to the membrane system analysis. The major technologies are i) cryogenic and ii) pressure swing adsorption. B. Adhikari et al. Separation and Purification Technology 268 (2021) 118703 Year 1 Cost Breakdown (US$1,638 K) 3.7% 1.0% 37.5% 57.9% Capital cost Energy cost Raw Materials cost Other operational cost (a) Capital cost breakdown (US$13,744 K) 15.6% 0.2% 30.2% 16.2% 3.8% 34.0% Membranes Membrane system installation Pump system installation Module materials Pump bare module Flow meter system (b) Fig. 11. The breakdown of (a) year 1 cost as well as (b) capital cost for a two staged system. 4.3.1. Cryogenic distillation Cryogenic air separation technology has been successfully employed for many years to supply oxygen for gasification of a wide range of hydrocarbon feedstocks yielding synthesis gas, which is then used to produce fuels, chemicals, and other valuable products [31]. In fact, cryogenic oxygen enrichment has been an industrial practice for more than a century. Cryogenic distillation consists of at least four major unit operations: air compression and purification, main heat exchanger, cryogenic distillation column, and product compression (internal or external). Air is compressed to 4-10 atm and then cooled to separate the component gases where oxygen is obtained at its boiling point, 90.2 K. Most of the cost for cryogenic distillation is incurred by cooling and compression needs. The major drawback of this technology is it best suited to larger applications due to long start up times, and as a result, the cost becomes high and undesirable for smaller scale systems, such as 5 MWe coal fired plants, whose needs may be more transient. The typical cost for this process reported in technical reports and in the literature is US$0.045/kg [32]. The typical O2 purity from this technology is well above 95%. 4.3.2. Pressure swing adsorption Pressure swing adsorption is a slightly more expensive process than cryogenic distillation. The reported production cost for a 90% 02 product is - US$0.065/kg [32]. Most of the cost is incurred with the 10 B. Adhikari et al. Separation and Purification Technology 268 (2021) 118703 Capital cost Breakdown (US$19,476 K) 0.1% 11.3% 14.2% 33.0% 4.1% 37.2% Membranes Module materials Pump bare module Membrane system installation Pump system installation Flow meter system (a) Year 1 Cost Breakdown (US$2,295 K) 2.6% 0.7% 35.1% 61.6% Capital cost Energy cost Raw Materials cost Other operational cost (b) Fig. 12. The breakdown of (a) year 1 cost as well as (b) capital cost for a three staged production scenario. B. Adhikari et al Separation and Purification Technology 268 (2021) 118703 References capital expenditure as well as to replace the adsorbents that are either lost or deactivated over time. The energy cost of the production is the largest portion of the operating cost. 4.4. Improvements of membrane based O2 enrichment technologies The cost of gas production can be improved by both improving the permeability and selectivity if permeability is not compromised by focusing on selectivity. However, there are certain practical limitations for both permeability and selectivity, and one is not mutually exclusive of the other (13,33). However, permeability and selectivity are not the only important considerations. For example, some membranes that have shown higher permeability and selectivity or have exciting levels of either characteristic, but also are difficult to manufacture at scale, may be impractical to deploy. 5. Summary Cost effective O/N2 separation is important for various industrial, medical, and agricultural processes. Modular, flexible, and continuous production of enriched O2 can be achieved by membrane separation. As the technology is emerging and evolving, different membranes with very attractive separation properties (high permeability and high selectivity) are becoming more available. As the permeance of the membrane increases with maintained selectivity, the production cost decreases very rapidly. However, that does not improve the product purity. In contrast, incrementing selec- tivity while keeping the permeance constant does not affect the cost of production, but the product purity improves. For a target purity, having the right selectivity and permeance is important for the optimized cost of the production. Generally, the membranes with high selectivities have lower permeance and vice versa and the practically achievable selec- tivity is 3.1-5.5 and permeance of 1000 GPU. A purity of 90% with one stage polymer membrane system is not practically achievable currently. Purity of 90% O2 can be achieved by using two and three stages, depending upon the type of membrane that is used. A membrane with the O2 permeance of 1000 GPU and selectivity of 5.5 in a two staged system, can produce 90% pure O2 at a cost of US $0.05/kg. This is less than the cost of enriched O2 produced using pressure swing adsorption technology but is more than what is achiev- able with cryogenic distillation technology. However, the economics for cryogenic distillation is for an optimized large-scale system and the cost would be expected to increase as the scale is decreased. Furthermore, flexibility of the systems also needs to be considered. Improving the membrane cost, cost of material used in membrane modules along with the improvement in both permeability and selectivity by not compro- mising one for the other can improve the cost of production significantly [1] ST. Summerfelt, B.J. Vinci, R.H. Piedrahita, Oxygenation and carbon dioxide control in water reuse systems, Aquacult. Eng. 22 (2000) 87-108. [2] L Shao, G. Xie, X. Liu, Y. Wu, J. Yu, Y. Wang Combustion behaviour and mechanism of a Cu-Ni. Mn alloy in an oxygen enriched atmosphere, Corros. Sci. 108253 (2019). [3] H. Wu, Y. Zhang, W. Chen, Z. Liu, T. Zhang, Q. Sun, 2. Zheng, Experimental research on the characteristics of ash in oxy fuel combustion, Fuel 263 (2020). 116799, 14) W. Zhang, S. Huang, S. Wu, Y. Wu, J. Gao, Study on the structure characteristics and gasification activity of residual carbon in biomass ashes obtained from different gasification technologies, Fuel 254 (2019), 115699. [S] Y. Gao, R. Gao, G. Zhang, Y. Zheng, J. Zhao, Oxidative desulfurization of model fuel in the presence of molecular oxygen over polyoxometalate based catalysts supported on carbon nanotubes, Fuel 224 (2018) 261-270. [6] J. Choi, J. Khim, B. Neppolian, Y. Son, Enhancement of sonochemical oxidation reactions using air sparging in a 36 kHz sonoreactor, Ultrason. Sonochem. 51 (2019) 412-418, [7] S.Y. Yoon, B.S. Chol, J.H. Ahn, T.S. Kim, Improvement of integrated gasification combined cycle performance using nitrogen from the air separation unit as turbine coolant, Appl. Therm. Eng. 151 (2019) 163-175, [8] V. Daniloska, P. Carretero, R. Tomovska, J.M, Asus, High performance pressure sensitive adhesives by miniemulsion photopolymerization in a continuous tubular reactor, Polymer 55 (2014) 5050 5056. 19) D.A. Sievers, E.M. Kuhn, J.J. Stickel, M.P. Tucker, EJ. Wolfrum, Online residence time distribution measurement of thermochemical biomass pretreatment reactors, Chem. Eng. Sci. 140 (2016) 330-336. [10] S. Haider, A. Lindbrathen, J.A. Lie, M.-B. Hags, Carbon membranes for oxygen enriched air - Part 11: Techno-economic analysis, Sep. Purif. Technol. 205 (2018) 251-262 [11] M. Bozorg, B. Addis, V. Piecialli, A. A. Ramirez-Santos, C. Castel, L. Pinn, E. Favre, Polymeric membrane materials for nitrogen production from air a process synthesis study, Chem. Eng. Sci. 207 (2019) 1196-1213. [12) AF Ismail, 02/N2 Separation, in: E. Drioli, L. Giomo (Eds.). Encyclopedia of Membranes, Springer, Berlin Heidelberg, Berlin, Heidelberg, 2016, pp. 1421-1422 [13] B,D, Freeman, Basis of permeability/selectivity tradeoff relations in polymerle gas separation membranes, Macromolecules 32 (1999) 375 380. (14) W.F. Castle, Air separation and liquefaction: recent developments and prospects for the beginning of the new millennium, Int. J. Refrig 25 (2002) 158-172. [15] W.P. Schmidt, K.S. Winegardner, M. Dennehy, H. Castle-Smith, Safe design and operation of a cryogenic air separation unit, Process Saf Prog 20 (2001) 269-279. [16] J. Miller, WL Luyben, S. Blouin, Economic incentive for intermittent operation of air separation plants with variable power costs, Ind Eng Chem Res 47 (2008) 1132-1139. [17] D. M. Ruthven, S. Farooq, Air separation by pressure swing adsorption, Gas Sep, Purif. 4 (1990) 141-148. [18] E. Alpay, C.N. Kenney, Adsorbent particle-size effects in the separation of air by rapid pressure swing adsorption, Chem Eng Sci 49 (1994) 3059-3075. [19] NO. tamcoff, Nitrogen separation from air by pressure swing adsorption Stud Surf Sei Catal 120 (1999) 347-370. [20] J.G. Jee, J.S. Lee, CH. Lee, Air separation by a small-scale two-bed medical 0-2 pressure swing adsorption, Ind Eng Chem Res 40 (2001) 3647-3658. [21] F.G. Wiesner, Basics and industrial applications of pressure swing adsorption (PSA), the modem way to separate gas, Gas Sep. Purif 2 (1988) 115-119. [22] N. Zhang, J. Xino, P. Benard, R. Chahine, Single and double bed pressure swing adsorption processes for H2/C0 syngas separation, Int J Hydrogen Energ 44 (2019) 26405-26418. [23] A. Moran, O. Talu, Limitations of portable pressure swing adsorption processes for air separation, Ind Eng Chem Res 57 (2018) 11981-11987. (24) R.S. Murall, T. Sankarshana, S. Sridhar, Air separation by polymer-based membrane technology, Sep Purif Rev 42 (2013) 130-186. [25] W.J. Koros, G.K. Meming, Membrane-based gas separation, J. Membrane Scl. 83 (1993) 1-80, [26] M. Freemantle, Membranes for gas separation, Chem. Eng. News 83 (2005) 49+ (27] H.B. Zhal, E.S. Rubin, Techno-economic assessment of polymer membrane systems for postcombustion carbon capture at coalfired power plants, Environ. Sci. Technol. 47 (2013) 3006 3014. [28] B. Hofs, J. Ogier, D. Vries, LF. Beerendonk, E.R. Cornelissen, Comparison of ceramic and polymeric membrane permeability and fouling using surface water, Sep. Purif. Technol. 79 (2011) 365 374 [29] S.S. Hosseini, S. Najari, P.K. Kundu, N.R. Tan, S.M. Roodashti, Simulation and sensitivity analysis of transport in asymmetric hollow fiber membrane permeators for air separation, RSC Adv. 5 (2015) 86359-86370, (30) K.C. Chong, S.O. Lal, H.S. Thiam, H.C. Teoh, S.L Heng, Recent progress of oxygen/ nitrogen separation using membrane technology, J. Eng. Sci. Technol. 11 (2016) 1016-1030. [31] A.R. Smith, J. Klosek. A review of air separation technologies and their integration with energy conversion processes, Fuel Process Technol. 70 (2001) 115-134. [32] B. Braunberger, A.R. Richard, V.K. Sethi, Low-cost Oxygen (LCO) for Small-scale Modular Gasification Systems, in: Thermosoly LLC, 2019, pp. Medium: ED. [33] H.F. Ye, D. LI, X. Ye, Y.G. Zheng, Z.Q. Zhang, H.W.Zhang, Z. Chen, An adjustable permeation membrane up to the separation for multicomponent gas mixture, Sci Rep Uk 9 (2019). Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Acknowledgments This manuscript has been authored by Battelle Energy Alliance, LLC under Contract No. DE-AC07-051D14517 with the U.S. Department of Energy. The views expressed in the article do not necessarily represent the views of the U.S. Department of Energy or the United States Gov- ernment. The U.S. Government retains and the publisher, by accepting the article for publication, acknowledges that the U.S. Government re- tains a nonexclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this work, or allow others to do so, for U.S. Government purposes. 12
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


