Answered step by step
Verified Expert Solution
Question
1 Approved Answer
MeO2(s)=Me(s)+O2(g) (Me: Metal MeO2 : Metal oxide) Gibbs free energy of the formation of one mole of pure MeO2 (s) from pure Me(s) and pure
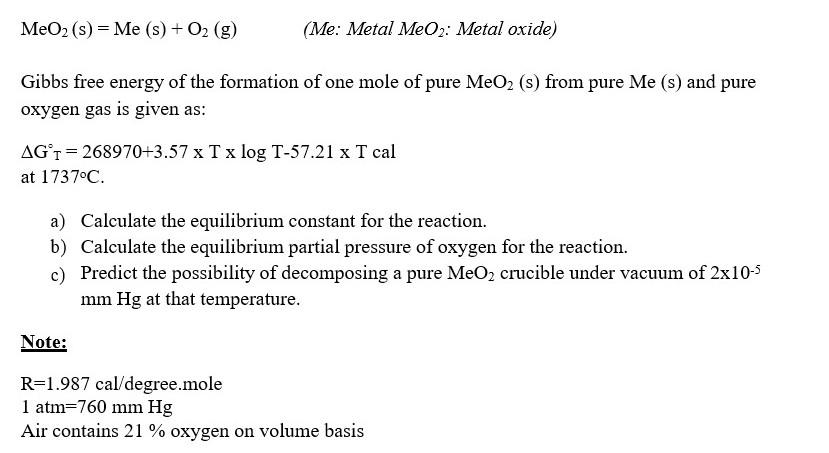
MeO2(s)=Me(s)+O2(g) (Me: Metal MeO2 : Metal oxide) Gibbs free energy of the formation of one mole of pure MeO2 (s) from pure Me(s) and pure oxygen gas is given as: GT=268970+3.57TlogT57.21T cal at 1737C. a) Calculate the equilibrium constant for the reaction. b) Calculate the equilibrium partial pressure of oxygen for the reaction. c) Predict the possibility of decomposing a pure MeO2 crucible under vacuum of 2105 mmHg at that temperature. Note: R=1.987cal/ degree.mole 1atm=760mmHg
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


