Answered step by step
Verified Expert Solution
Question
1 Approved Answer
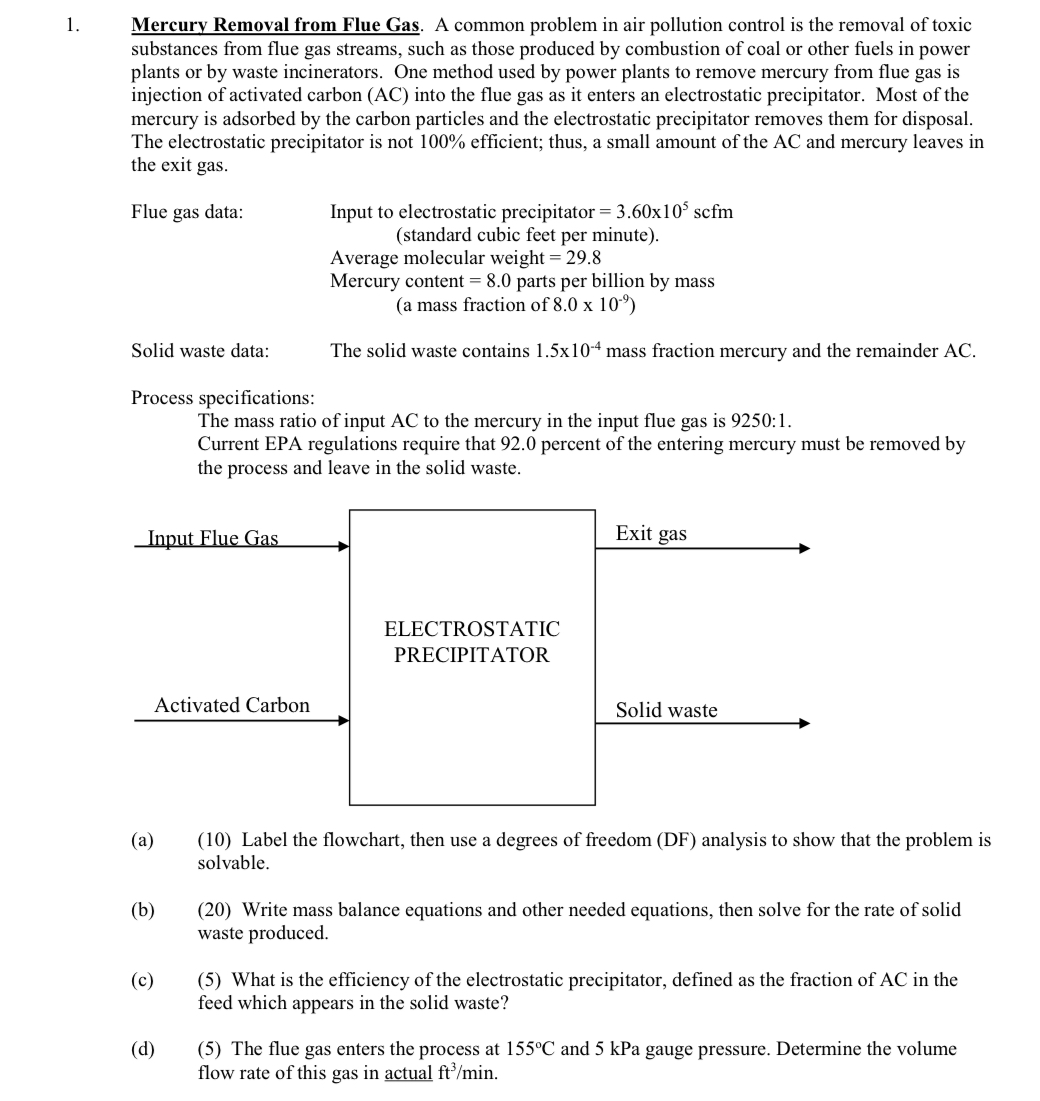
Mercury Removal from Flue Gas. A common problem in air pollution control is the removal of toxic substances from flue gas streams, such as those
Mercury Removal from Flue Gas. A common problem in air pollution control is the removal of toxic substances from flue gas streams, such as those produced by combustion of coal or other fuels in power plants or by waste incinerators. One method used by power plants to remove mercury from flue gas is injection of activated carbon AC into the flue gas as it enters an electrostatic precipitator. Most of the mercury is adsorbed by the carbon particles and the electrostatic precipitator removes them for disposal. The electrostatic precipitator is not efficient; thus, a small amount of the and mercury leaves in the exit gas.
Flue gas data:
Input to electrostatic precipitator scfm standard cubic feet per minute
Average molecular weight
Mercury content parts per billion by mass
a mass fraction of
Solid waste data: The solid waste contains mass fraction mercury and the remainder AC
Process specifications:
The mass ratio of input AC to the mercury in the input flue gas is :
Current EPA regulations require that percent of the entering mercury must be removed by the process and leave in the solid waste.
a Label the flowchart, then use a degrees of freedom DF analysis to show that the problem is solvable.
b Write mass balance equations and other needed equations, then solve for the rate of solid waste produced.
c What is the efficiency of the electrostatic precipitator, defined as the fraction of AC in the feed which appears in the solid waste?
d The flue gas enters the process at and kPa gauge pressure. Determine the volume flow rate of this gas in actual
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


