Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Methane and carbon dioxide generation by anaerobic digestion can be calculated using the following equation: CaHbbOcNd+(44ab2c+3d)H2O(84a+b2c3d)CH4+(84ab+2c+3d)CO2+dN3 Given the chemical composition of ethanol C2H5OH, propionic acid
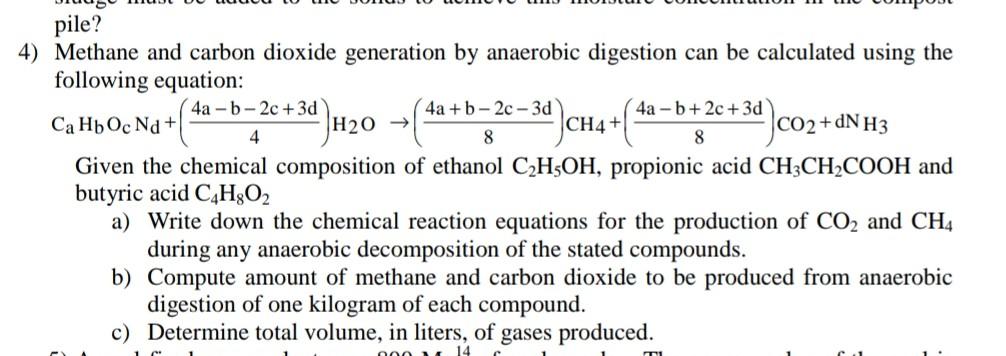

Methane and carbon dioxide generation by anaerobic digestion can be calculated using the following equation: CaHbbOcNd+(44ab2c+3d)H2O(84a+b2c3d)CH4+(84ab+2c+3d)CO2+dN3 Given the chemical composition of ethanol C2H5OH, propionic acid CH3CH2COOH and butyric acid C4H8O2 a) Write down the chemical reaction equations for the production of CO2 and CH4 during any anaerobic decomposition of the stated compounds. b) Compute amount of methane and carbon dioxide to be produced from anaerobic digestion of one kilogram of each compound. c) Determine total volume, in liters, of gases produced. 7) A refractory combustion unit lined with no water wall and no heat recovery is burning refuse-derived fuel (RDF) consisting of 85% organics, 10\% water, \& 5\% inorganics or inerts at a rate of 950kg/hr. Determine the temperature, T, at which ash exits the combustion chamber (in both C and F ), assuming the following: Heat value of the fuel =19,000kJ/kg on a moisture-free basis. Air flow =9,500kg/h& that the under- \& overfire air contributes negligible heat. 5% of the heat input is lost due to radiation 15% of the fuel remains uncombusted in the ash Specific heat of ash =0.837kJ/kg/C, Specific heat of air is 1.0kJ/kg/C. Latent heat of vaporization =2575kJ/kg. Temperature of the stack gases =1500C
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


