Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Determine the amount of heat removed from the reactor per mole of CH4 fed to the reactor. 2. Methane and oxygen at 25C are fed
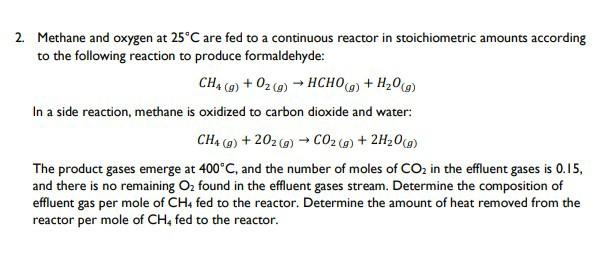
Determine the amount of heat removed from the reactor per mole of CH4 fed to the reactor.
2. Methane and oxygen at 25C are fed to a continuous reactor in stoichiometric amounts according to the following reaction to produce formaldehyde: CH4 (g) + O2(g) HCHO(g) + HO(g) In a side reaction, methane is oxidized to carbon dioxide and water: CH4 (g) +202 (g) CO (g) + 2HO(g) The product gases emerge at 400C, and the number of moles of CO in the effluent gases is 0.15, and there is no remaining O found in the effluent gases stream. Determine the composition of effluent gas per mole of CH4 fed to the reactor. Determine the amount of heat removed from the reactor per mole of CH4 fed to the reactor.
Step by Step Solution
★★★★★
3.38 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1
0 CHY 20 25C CHyzl with Imole CO with 015 mole cos 102385 HO 210 4100C 3 I mole o...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


