Question
Methane enters a turbine at T=600K, P = 1MPa and leaves at T=400K and P=0.2Mpa. How much work is produced for each mole of
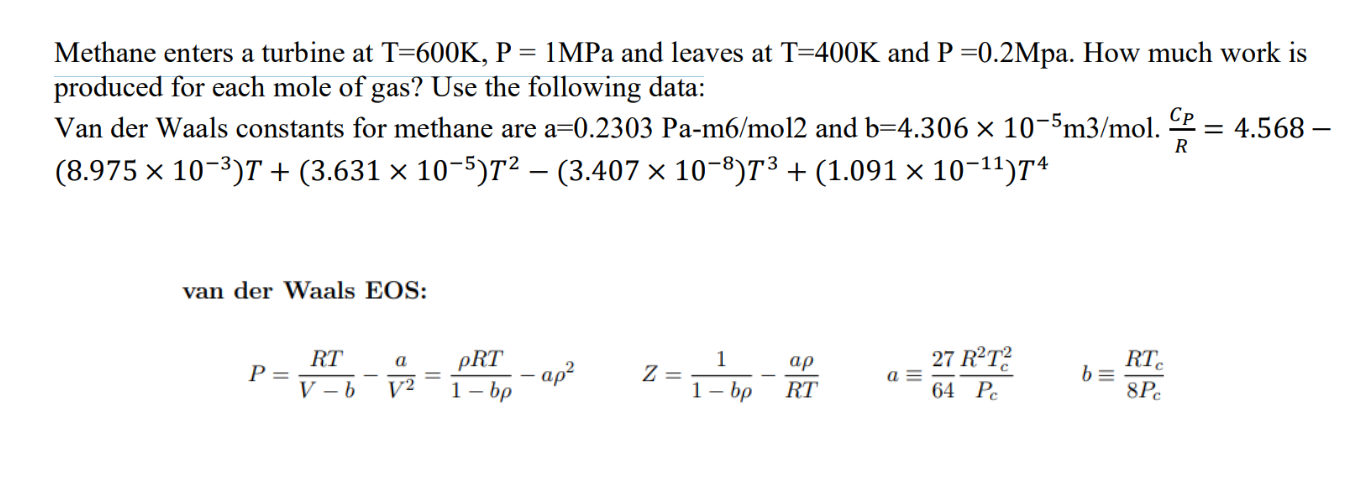
Methane enters a turbine at T=600K, P = 1MPa and leaves at T=400K and P=0.2Mpa. How much work is produced for each mole of gas? Use the following data: Van der Waals constants for methane are a=0.2303 Pa-m6/mol2 and b=4.306 10-5m3/mol. (8.975 10)T + (3.631 105)T (3.407 108)T + (1.091 10-11)74 R = 4.568- van der Waals EOS: . a PRT 1 P = - ap Z = a= V-b V2 1-bp 1-bp RT 27 R2T2 64 Pc RTC b = 8PC
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals Of Chemical Engineering Thermodynamics
Authors: Kevin D. Dahm, Donald P. Visco
1st Edition
1111580707, 978-1111580704
Students also viewed these Algorithms questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



