Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Methyl ethyl ketone (MEK) can be produced from the dehydrogenation of butanol (Bu) over a zinc oxide catalyst according to the following equation. ButanolMEK+H2 The
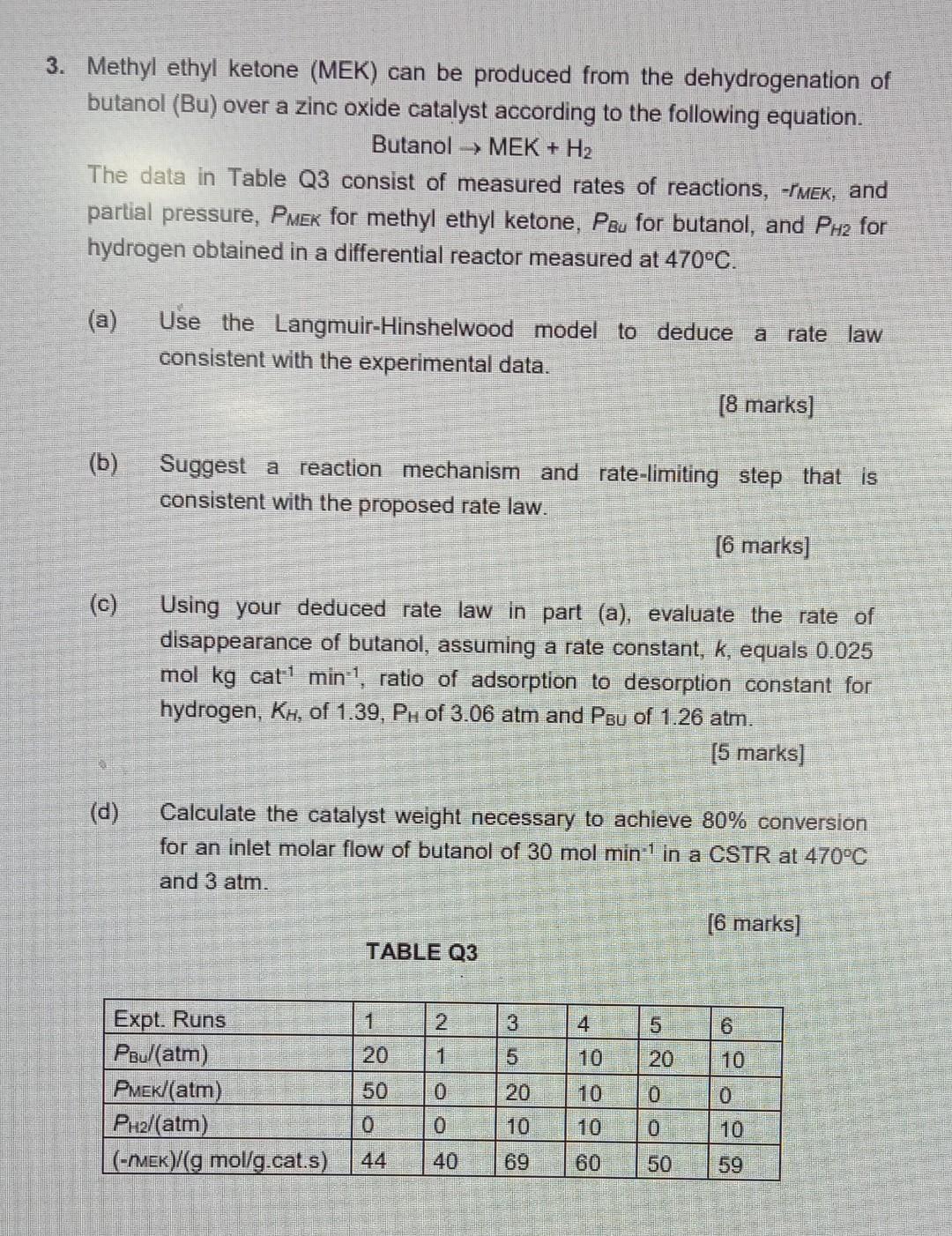
Methyl ethyl ketone (MEK) can be produced from the dehydrogenation of butanol (Bu) over a zinc oxide catalyst according to the following equation. ButanolMEK+H2 The data in Table Q3 consist of measured rates of reactions, rMEK, and partial pressure, PMEK for methyl ethyl ketone, PBu for butanol, and PH2 for hydrogen obtained in a differential reactor measured at 470C. (a) Use the Langmuir-Hinshelwood model to deduce a rate law consistent with the experimental data. [8 marks] (b) Suggest a reaction mechanism and rate-limiting step that is consistent with the proposed rate law. [6 marks] (c) Using your deduced rate law in part (a), evaluate the rate of disappearance of butanol, assuming a rate constant, k, equals 0.025 hydrogen, KH, of 1.39,PH of 3.06atm and PBU of 1.26atm. [5 marks] (d) Calculate the catalyst weight necessary to achieve 80% conversion for an inlet molar flow of butanol of 30molmin1 in a CSTR at 470C and 3atm. [6 marks] TABLE Q3 Methyl ethyl ketone (MEK) can be produced from the dehydrogenation of butanol (Bu) over a zinc oxide catalyst according to the following equation. ButanolMEK+H2 The data in Table Q3 consist of measured rates of reactions, rMEK, and partial pressure, PMEK for methyl ethyl ketone, PBu for butanol, and PH2 for hydrogen obtained in a differential reactor measured at 470C. (a) Use the Langmuir-Hinshelwood model to deduce a rate law consistent with the experimental data. [8 marks] (b) Suggest a reaction mechanism and rate-limiting step that is consistent with the proposed rate law. [6 marks] (c) Using your deduced rate law in part (a), evaluate the rate of disappearance of butanol, assuming a rate constant, k, equals 0.025 hydrogen, KH, of 1.39,PH of 3.06atm and PBU of 1.26atm. [5 marks] (d) Calculate the catalyst weight necessary to achieve 80% conversion for an inlet molar flow of butanol of 30molmin1 in a CSTR at 470C and 3atm. [6 marks] TABLE Q3
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


