Answered step by step
Verified Expert Solution
Question
1 Approved Answer
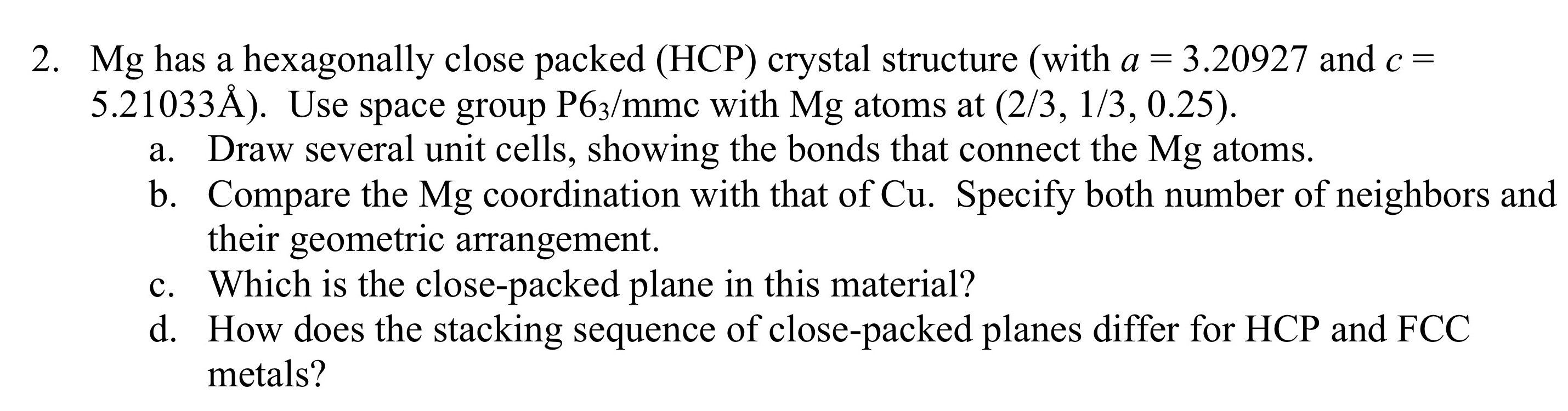
Mg has a hexagonally close packed ( HCP ) crystal structure ( with a = 3 . 2 0 9 2 7 and c =
Mg has a hexagonally close packed HCP crystal structure with and
Use space group with atoms at
a Draw several unit cells, showing the bonds that connect the atoms.
b Compare the coordination with that of Specify both number of neighbors and
their geometric arrangement.
c Which is the closepacked plane in this material?
d How does the stacking sequence of closepacked planes differ for HCP and FCC
metals?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


