Answered step by step
Verified Expert Solution
Question
1 Approved Answer
missed the lab day NAME DATE REPORT FOR EXPERIMENT 23 SECTION INSTRUCTOR Neutralization-Titration II A Molarity of an Unknown Acid Data Table Sample 3 Gif
missed the lab day 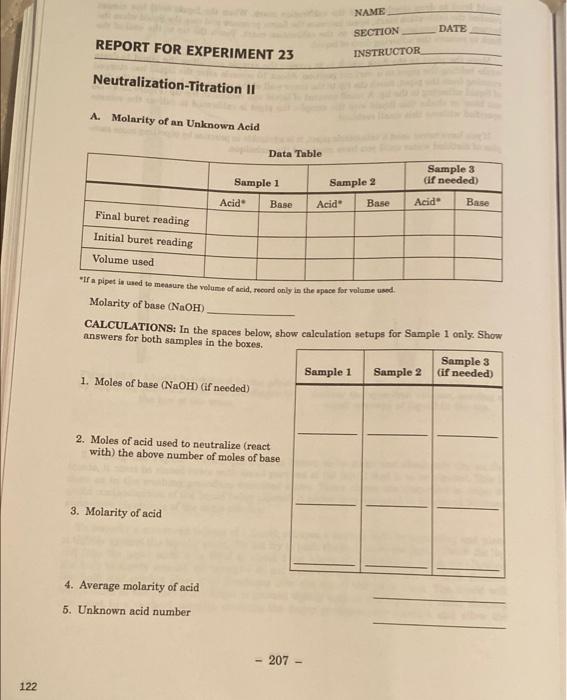
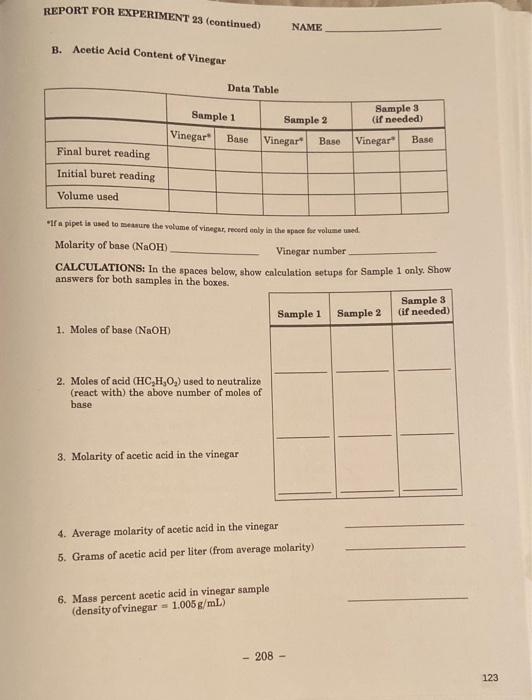
NAME DATE REPORT FOR EXPERIMENT 23 SECTION INSTRUCTOR Neutralization-Titration II A Molarity of an Unknown Acid Data Table Sample 3 Gif needed) Sample 1 Sample 2 Acid Base Acid" Base Acid Base Final buret reading Initial buret reading Volume used "a pipet la cured to measure the volume of acid, record only in the space for volume used Molarity of base (NaOH) CALCULATIONS: In the spaces below, show calculation setups for Sample 1 only. Show answers for both samples in the boxes. Sample 3 Sample 1 Sample 2 (if needed) 1. Moles of base (NaOH) (if needed) 2. Moles of acid used to neutralize (react with the above number of moles of base 3. Molarity of acid 4. Average molarity of acid 5. Unknown acid number - 207 - 122 REPORT FOR EXPERIMENT 23 (continued) NAME B. Acetic Acid Content of Vinegar Data Table Sample 3 (if needed) Sample 1 Vinegar Base Sample 2 Vinegar Base Vinegar Base Final buret reading Initial buret reading Volume used "If a pipet is used to make the volume of vinegar, record only in the space for volume wed. Molarity of base (NaOH) Vinegar number CALCULATIONS: In the spaces below, show calculation setups for Sample 1 only. Show answers for both samples in the boxes. Sample 3 Sample 1 Sample 2 (if needed) 1. Moles of base (NaOH) 2. Moles of acid (HC,H,O,) used to neutralize (react with the above number of moles of base 3. Molarity of acetic acid in the vinegar 4. Average molarity of acetic acid in the vinegar 5. Grams of acetic acid per liter (from average molarity) 6. Mass percent acetic acid in vinegar sample (density of vinegar 1.005 g/mL) 208 - 123 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


