Answered step by step
Verified Expert Solution
Question
1 Approved Answer
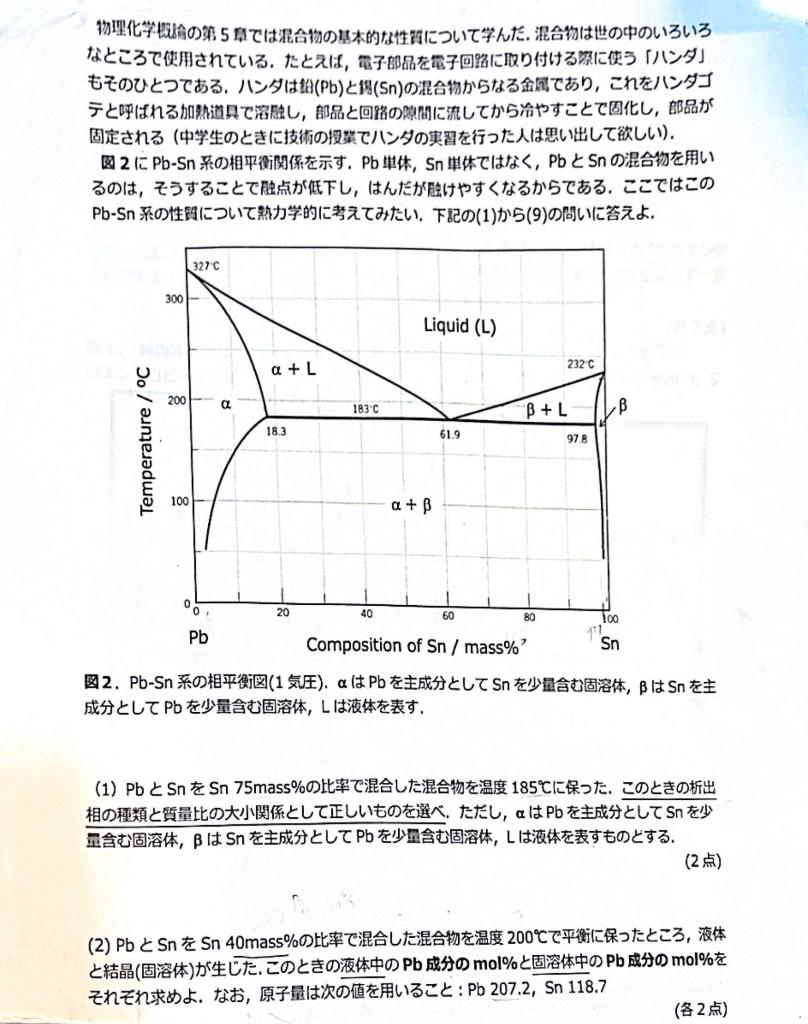
Mixtures are used in various places around the world. Solder is one of them. Solder is a mixture of lead (Pb) and (Sn), which is
Mixtures are used in various places around the world. Solder is one of them. Solder is a mixture of lead (Pb) and (Sn), which is melted with a heating tool called a soldering iron. , the parts are fixed (remember those who practiced soldering in technology class when they were junior high school students). Figure 2 shows the phase relationship of the pl-Sn system. The reason why a mixture of Pb and Sn is used instead of single Pb or single Sn is that this lowers the melting point and makes it easier to touch the solder. 1) mixture of p and Sn at a ratio of 75 mass% Sn was kept at 185C. At this time, choose the correct one for the type of precipitate phase and the magnitude relationship between the mass ratios, where is a solid solution containing Pb as the main component and a small amount of Sn, B is a solid solution containing Sn as the main component and a small amount of PD, and L is taken to represent a liquid.
(2) When the mixture of p and Sn in a ratio of 40 mass% Sn was equilibrated at 200C, a liquid and a crystal (solid solution) formed. Find the mol% of the Pb component in the liquid and the mol% of the Pb component in the solid solution at this time. Use the following atomic weights: Pb 207.2, Sn 118.7
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


