

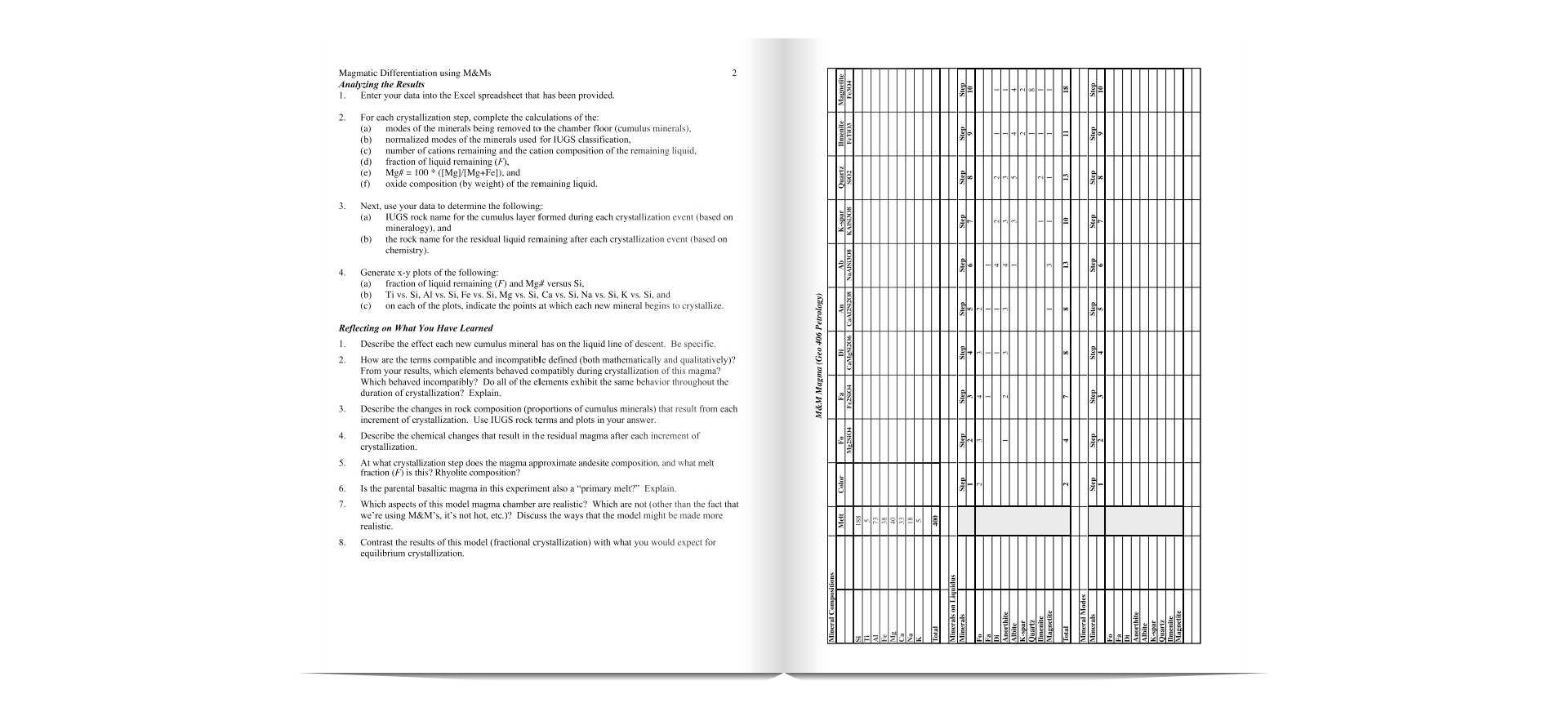
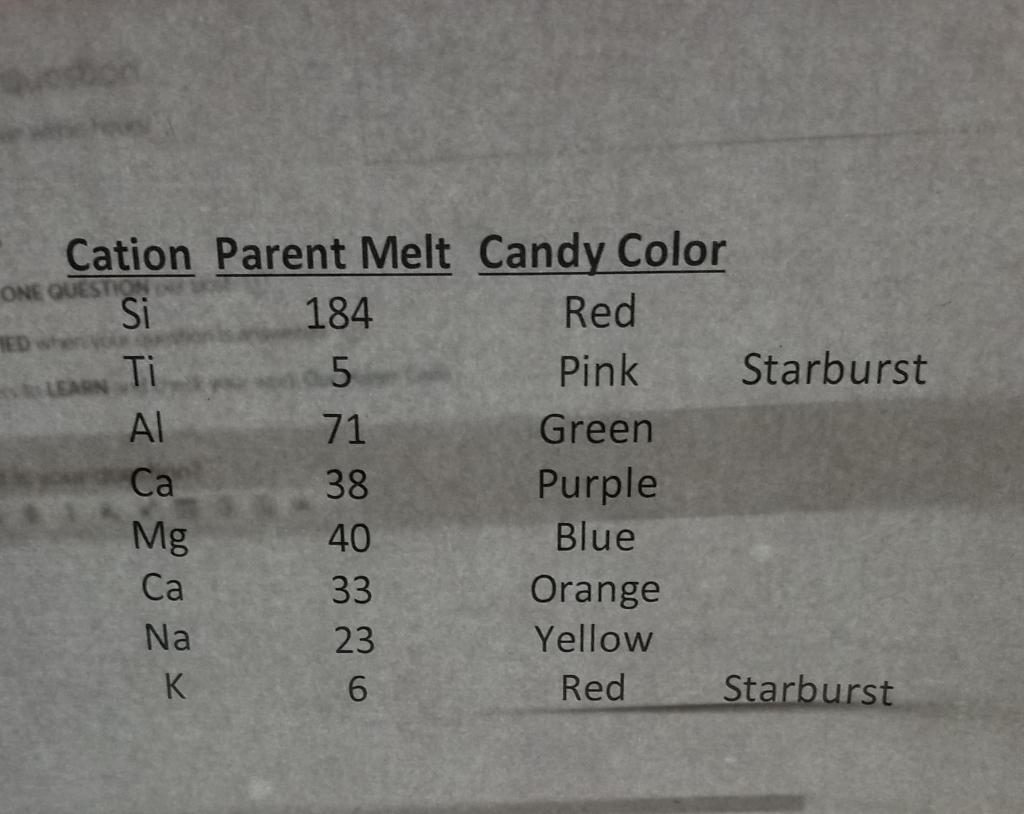
M&M Magma (Geo 406 Petrology) TUGS Nornis & Mineral Compositions Classification DIN plan ples An in Plag M&M's Remaining Elements AI Fe 40 Ca 400 Total Liq TIMI Liquid Composition Parent 1.251 18.25 9.50 10.00 Na 4.50 1.251 Total 100.00 1.001 0.51 M&M Magma (Geo 406 Petrology) Liquid Composition (oxide wt) Oxides Mol. Wt Parent 6008 TiO. 79.87 1.00 85.96 71.84 6.83 Mgo 30 4.03! Cao 5008 4.63 Na o 61.98 0.591 Total: 9.537 Liquid Composition (oxide wt Oxides Parent 51.72 1439 110, ALO Peo MgO Cao Mao 12.51 2.56 1.08 1001 Total: a20+ K20 LAS Class. 3.641 basalti Magmatic Differentiation using M&Ms 1 HANDOUT Differentiation of Magmas By Fractional Crystallization Objective The objective of this exercise is to gain first-hand knowledge of the process of magmatic differentiation by fractional crystallization. The activity also provides an opportunity to utilize your knowledge of mineral stoichiometry, the IUGS classification system, and spreadsheets. A Bit of History and Terminology In the introductory geology course you learned about Bowen's reaction series and the importance of crystal-melt fractionation in generating the spectrum of observed igneous rock compositions (e.g., basalt, andesite, rhyolite). Magmatic differentiation is the process by which diverse rock types are generated from a single magma. Differentiation is accomplished by crystal-melt fractionation, a two- stage process that involves the formation and mechanical separation of compositionally distinct phases. In 1844 Charles Darwin described flows from the Galpagos Islands in which the lowest flows contained greater proportions of feldspar crystals. These observations led Darwin to propose that density differences between crystals and melt would result in mechanical separation of these two phases and the formation of different magma types. This process, known today as gravity settling, was the focus of detailed experimental studies by N.L. Bowen. Today, several additional mechanisms of crystal-melt fractionation are also recognized, including: flow segregation, filter pressing, and convective melt fractionation. Note: This exercise was altered a bit from one created by Karl Wirth. Constructing the Magma Chamber 1 In this exercise each major cation (e.g., Si, Ti, Al) will be represented by a different colored M&M (e.g., brown, purple, red, respectively). Count out the appropriate number of M&M's for each cation (refer to data sheet). Mix the M&M's and pile them in the magma chamber (circled area on large white sheet of paper). Crystallization and Fractionation of the Magma 1. Before you begin, note the general proportions of the different cations (colors) in the magma chamber. Determine the composition and stoichiometry of each of the minerals involved in the crystallization process. For first increment of crystallization, move the appropriate number of M&M's from the magma chamber to the "floor" of the magma chamber. For each cation, record the number that remains in the magma chamber. 2. For each increment of crystallization, move the appropriate number of cations (M&M's) from the chamber to the floor of the chamber. Note: it is helpful to group the cations that were removed in each crystallization step in separate layers. In other words, move the M&M's that were crystallized during the first step the furthest away from the magma chamber, cations from cach additional crystallization step will be successively closer to the magma chamber. 3. After each crystallization step, you should observe the proportions of the cations (colored M&M's) in the remaining liquid and in the cumulus layers. Record the number of remaining M&M's in each step. 4. Before your experiment dismantled (or consumed), describe the general trends that you observed during the fractional crystallization of the magma? 2 Magmatic Differentiation using M&M Analyzing the Results 1. Enter your data into the Excel spreadsheet that has been provided. For each crystallization step, complete the calculations of the modes of the minerals being removed to the chamber floor (cumulus minerals), (b) normalized modes of the minerals used for IUGS classification, (c) number of cations remaining and the cation composition of the remaining liquid, (d) fraction of liquid remaining (F). (e) Mg# = 100 * ([Mg]/[Mg+Fel), and oxide composition (by weight) of the remaining liquid. 3. Next, use your data to determine the following: (a) IUGS rock name for the cumulus layer formed during each crystallization event (based on mineralogy), and (b) the rock name for the residual liquid remaining after each crystallization event (based on chemistry) 16 El 4 Generate x-y plots of the following: (a) fraction of liquid remaining (F) and Mg# versus Si, (b) Ti vs. Si. Al vs. Si, Fe vs. Si, Mg vs. Si, Ca vs. Si, Na vs. Si, K vs. Si, and (c) on each of the plots, indicate the points at which each new mineral begins to crystallize. Reflecting on What You Have Learned 1. Describe the effect each new cumulus mineral has on the liquid line of descent. Be specific. 2. How are the terms compatible and incompatible defined (both mathematically and qualitatively)? From your results, which elements behaved compatibly during crystallization of this magma? Which behaved incompatibly? Do all of the elements exhibit the same behavior throughout the duration of crystallization? Explain. 3. Describe the changes in rock composition (proportions of cumulus minerals) that result from each increment of crystallization. Use IUGS rock terms and plots in your answer. 4. Describe the chemical changes that result in the residual magma after each increment of crystallization At what crystallization step does the magma approximate andesite composition, and what melt fraction (F) is this? Rhyolite composition? Is the parental basaltic magma in this experiment also a "primary melt?" Explain. 7. Which aspects of this model magma chamber are realistic? Which are not (other than the fact that we're using M&M's, it's not hot, etc.)? Discuss the ways that the model might be made more realistic. Contrast the results of this model (fractional crystallization) with what you would expect for equilibrium crystallization, M&M Magma (Geo 406 Petrology) 5 6. 8. ONE QUESTION LEARN Cation Parent Melt Candy Color Si 184 Red Ti 5 Pink Starburst 71 Green 38 Purple 40 Blue Ca 33 Orange Na 23 Yellow K 6 Red Starburst Mg M&M Magma (Geo 406 Petrology) TUGS Nornis & Mineral Compositions Classification DIN plan ples An in Plag M&M's Remaining Elements AI Fe 40 Ca 400 Total Liq TIMI Liquid Composition Parent 1.251 18.25 9.50 10.00 Na 4.50 1.251 Total 100.00 1.001 0.51 M&M Magma (Geo 406 Petrology) Liquid Composition (oxide wt) Oxides Mol. Wt Parent 6008 TiO. 79.87 1.00 85.96 71.84 6.83 Mgo 30 4.03! Cao 5008 4.63 Na o 61.98 0.591 Total: 9.537 Liquid Composition (oxide wt Oxides Parent 51.72 1439 110, ALO Peo MgO Cao Mao 12.51 2.56 1.08 1001 Total: a20+ K20 LAS Class. 3.641 basalti Magmatic Differentiation using M&Ms 1 HANDOUT Differentiation of Magmas By Fractional Crystallization Objective The objective of this exercise is to gain first-hand knowledge of the process of magmatic differentiation by fractional crystallization. The activity also provides an opportunity to utilize your knowledge of mineral stoichiometry, the IUGS classification system, and spreadsheets. A Bit of History and Terminology In the introductory geology course you learned about Bowen's reaction series and the importance of crystal-melt fractionation in generating the spectrum of observed igneous rock compositions (e.g., basalt, andesite, rhyolite). Magmatic differentiation is the process by which diverse rock types are generated from a single magma. Differentiation is accomplished by crystal-melt fractionation, a two- stage process that involves the formation and mechanical separation of compositionally distinct phases. In 1844 Charles Darwin described flows from the Galpagos Islands in which the lowest flows contained greater proportions of feldspar crystals. These observations led Darwin to propose that density differences between crystals and melt would result in mechanical separation of these two phases and the formation of different magma types. This process, known today as gravity settling, was the focus of detailed experimental studies by N.L. Bowen. Today, several additional mechanisms of crystal-melt fractionation are also recognized, including: flow segregation, filter pressing, and convective melt fractionation. Note: This exercise was altered a bit from one created by Karl Wirth. Constructing the Magma Chamber 1 In this exercise each major cation (e.g., Si, Ti, Al) will be represented by a different colored M&M (e.g., brown, purple, red, respectively). Count out the appropriate number of M&M's for each cation (refer to data sheet). Mix the M&M's and pile them in the magma chamber (circled area on large white sheet of paper). Crystallization and Fractionation of the Magma 1. Before you begin, note the general proportions of the different cations (colors) in the magma chamber. Determine the composition and stoichiometry of each of the minerals involved in the crystallization process. For first increment of crystallization, move the appropriate number of M&M's from the magma chamber to the "floor" of the magma chamber. For each cation, record the number that remains in the magma chamber. 2. For each increment of crystallization, move the appropriate number of cations (M&M's) from the chamber to the floor of the chamber. Note: it is helpful to group the cations that were removed in each crystallization step in separate layers. In other words, move the M&M's that were crystallized during the first step the furthest away from the magma chamber, cations from cach additional crystallization step will be successively closer to the magma chamber. 3. After each crystallization step, you should observe the proportions of the cations (colored M&M's) in the remaining liquid and in the cumulus layers. Record the number of remaining M&M's in each step. 4. Before your experiment dismantled (or consumed), describe the general trends that you observed during the fractional crystallization of the magma? 2 Magmatic Differentiation using M&M Analyzing the Results 1. Enter your data into the Excel spreadsheet that has been provided. For each crystallization step, complete the calculations of the modes of the minerals being removed to the chamber floor (cumulus minerals), (b) normalized modes of the minerals used for IUGS classification, (c) number of cations remaining and the cation composition of the remaining liquid, (d) fraction of liquid remaining (F). (e) Mg# = 100 * ([Mg]/[Mg+Fel), and oxide composition (by weight) of the remaining liquid. 3. Next, use your data to determine the following: (a) IUGS rock name for the cumulus layer formed during each crystallization event (based on mineralogy), and (b) the rock name for the residual liquid remaining after each crystallization event (based on chemistry) 16 El 4 Generate x-y plots of the following: (a) fraction of liquid remaining (F) and Mg# versus Si, (b) Ti vs. Si. Al vs. Si, Fe vs. Si, Mg vs. Si, Ca vs. Si, Na vs. Si, K vs. Si, and (c) on each of the plots, indicate the points at which each new mineral begins to crystallize. Reflecting on What You Have Learned 1. Describe the effect each new cumulus mineral has on the liquid line of descent. Be specific. 2. How are the terms compatible and incompatible defined (both mathematically and qualitatively)? From your results, which elements behaved compatibly during crystallization of this magma? Which behaved incompatibly? Do all of the elements exhibit the same behavior throughout the duration of crystallization? Explain. 3. Describe the changes in rock composition (proportions of cumulus minerals) that result from each increment of crystallization. Use IUGS rock terms and plots in your answer. 4. Describe the chemical changes that result in the residual magma after each increment of crystallization At what crystallization step does the magma approximate andesite composition, and what melt fraction (F) is this? Rhyolite composition? Is the parental basaltic magma in this experiment also a "primary melt?" Explain. 7. Which aspects of this model magma chamber are realistic? Which are not (other than the fact that we're using M&M's, it's not hot, etc.)? Discuss the ways that the model might be made more realistic. Contrast the results of this model (fractional crystallization) with what you would expect for equilibrium crystallization, M&M Magma (Geo 406 Petrology) 5 6. 8. ONE QUESTION LEARN Cation Parent Melt Candy Color Si 184 Red Ti 5 Pink Starburst 71 Green 38 Purple 40 Blue Ca 33 Orange Na 23 Yellow K 6 Red Starburst Mg










