Answered step by step
Verified Expert Solution
Question
1 Approved Answer
MN Chemicals, always on the cutting edge of catalysis and reactor engineering, has developed a new catalyst for converting dirt (D) to cash (C).
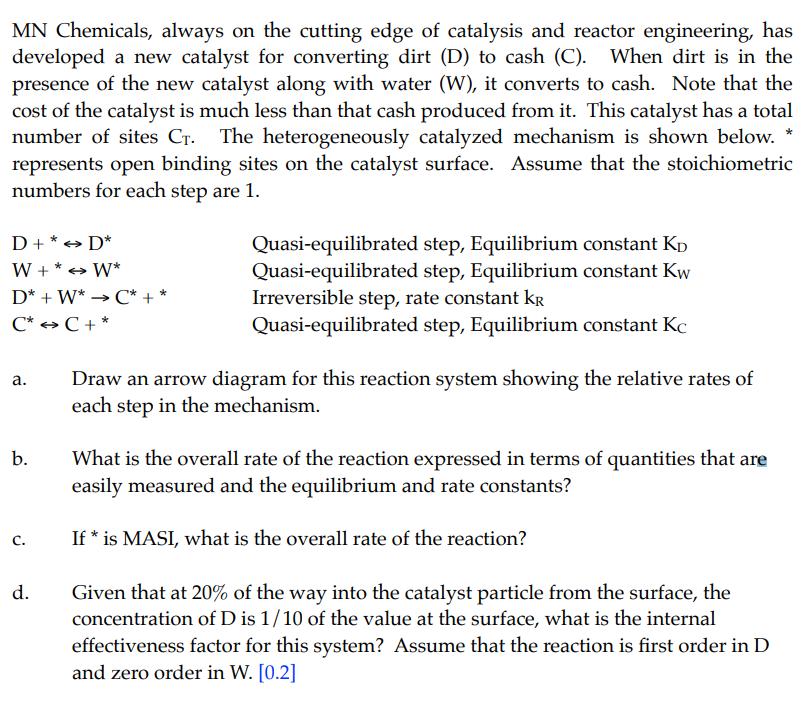
MN Chemicals, always on the cutting edge of catalysis and reactor engineering, has developed a new catalyst for converting dirt (D) to cash (C). When dirt is in the presence of the new catalyst along with water (W), it converts to cash. Note that the cost of the catalyst is much less than that cash produced from it. This catalyst has a total number of sites CT. The heterogeneously catalyzed mechanism is shown below. represents open binding sites on the catalyst surface. Assume that the stoichiometric numbers for each step are 1. D+* D* W+* W* D* W* C* + * C* C + a. b. C. d. ->>> -> Quasi-equilibrated step, Equilibrium constant KD Quasi-equilibrated step, Equilibrium constant Kw Irreversible step, rate constant kr Quasi-equilibrated step, Equilibrium constant Kc Draw an arrow diagram for this reaction system showing the relative rates of each step in the mechanism. What is the overall rate of the reaction expressed in terms of quantities that are easily measured and the equilibrium and rate constants? If * is MASI, what is the overall rate of the reaction? Given that at 20% of the way into the catalyst particle from the surface, the concentration of D is 1/10 of the value at the surface, what is the internal effectiveness factor for this system? Assume that the reaction is first order in D and zero order in W. [0.2]
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


