Question
Model Gasoline as Octane (CH8) 18 Model air as 3.76 moles of Nitrogen (N) for every 1 mole of Oxygen (0) With these assumptions
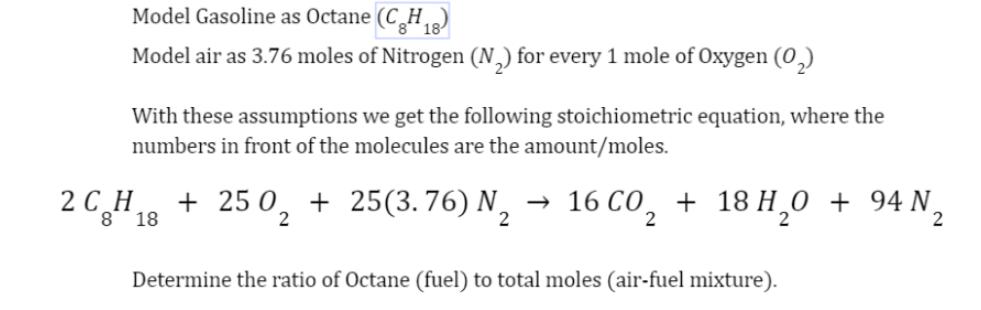
Model Gasoline as Octane (CH8) 18 Model air as 3.76 moles of Nitrogen (N) for every 1 mole of Oxygen (0) With these assumptions we get the following stoichiometric equation, where the numbers in front of the molecules are the amount/moles. 2 CH 18 8 + 250, +25(3.76) N 16C0, + 18H,0 + 94N, 2 2 Determine the ratio of Octane (fuel) to total moles (air-fuel mixture).
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The stoichiometric equation for the combustion of octane C8H18 with oxygen O2 and ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Practical Management Science
Authors: Wayne L. Winston, Christian Albright
5th Edition
1305631540, 1305631544, 1305250907, 978-1305250901
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



