Answered step by step
Verified Expert Solution
Question
1 Approved Answer
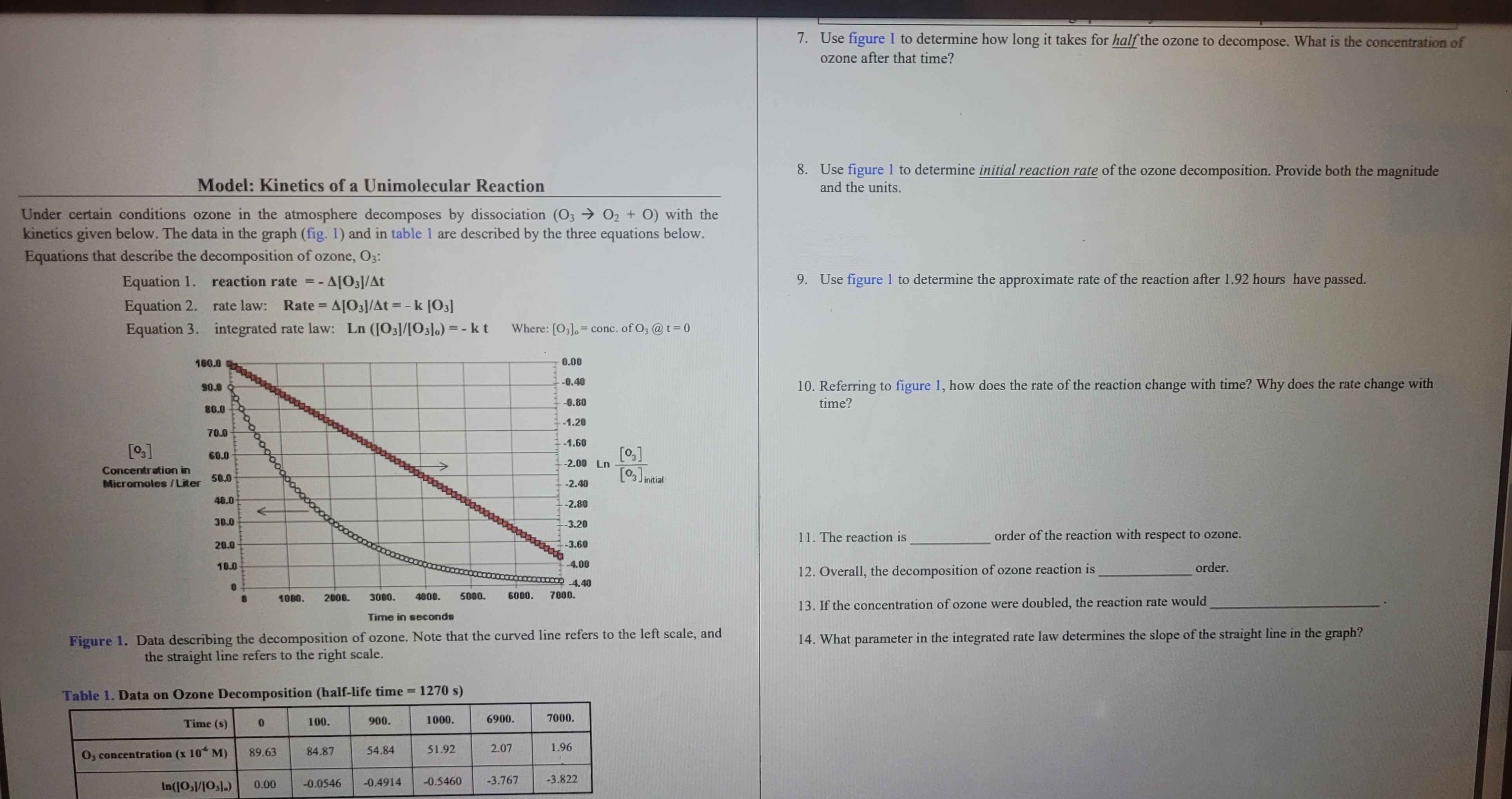
Model: Kinetics of a Unimolecular Reaction Under certain conditions ozone in the atmosphere decomposes by dissociation ( O 3 O 2 + O ) with
Model: Kinetics of a Unimolecular Reaction
Under certain conditions ozone in the atmosphere decomposes by dissociation with the
kinetics given below. The data in the graph fig and in table are described by the three equations below.
Equations that describe the decomposition of ozone, :
Equation reaction rate
Equation rate law: Rate
Equation integrated rate law: Where: conc. of @
Use figure to determine how long it takes for half the ozone to decompose. What is the concentration of
ozone after that time?
Use figure to determine initial reaction rate of the ozone decomposition. Provide both the magnitude
and the units.
Use figure to determine the approximate rate of the reaction after hours have passed.
Referring to figure how does the rate of the reaction change with time? Why does the rate change with
time?
The reaction is
order of the reaction with respect to ozone.
Overall, the decomposition of ozone reaction i
If the concentration of ozone were doubled, the reaction rate would
What parameter in the integrated rate law determines the slope of the straight line in the graph?
Table Data on Ozone Decomposition halflife time
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


