Answered step by step
Verified Expert Solution
Question
1 Approved Answer
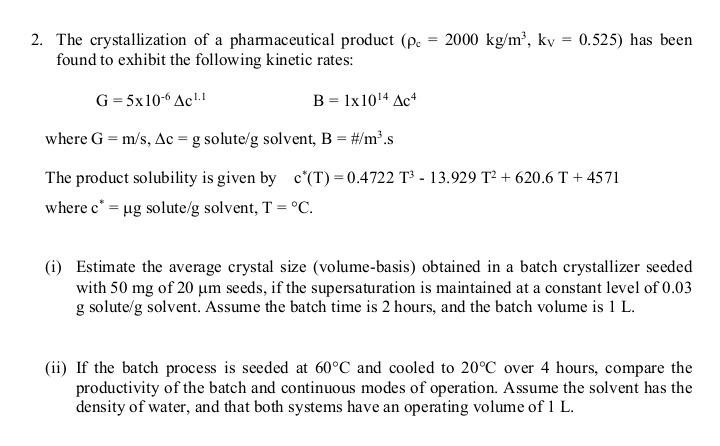
Module: Advanced Separation Processes 2. The crystallization of a pharmaceutical product ( c=2000kg/m3,kV=0.525 ) has been found to exhibit the following kinetic rates: G=5106c1.1B=11014c4 where
Module: Advanced Separation Processes
2. The crystallization of a pharmaceutical product ( c=2000kg/m3,kV=0.525 ) has been found to exhibit the following kinetic rates: G=5106c1.1B=11014c4 where G=m/s,c=g solute /g solvent, B=#/m3s The product solubility is given by c(T)=0.4722T313.929T2+620.6T+4571 where c= g solute/g solvent, T=C. (i) Estimate the average crystal size (volume-basis) obtained in a batch crystallizer seeded with 50mg of 20m seeds, if the supersaturation is maintained at a constant level of 0.03 g solute/g solvent. Assume the batch time is 2 hours, and the batch volume is 1L. (ii) If the batch process is seeded at 60C and cooled to 20C over 4 hours, compare the productivity of the batch and continuous modes of operation. Assume the solvent has the density of water, and that both systems have an operating volume of 1LStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


