Question
Mount Rainier in Washington has a height of 4400 m above sea level. Atmospheric pressure decreases with altitude by P = Pe 8MhRT ,
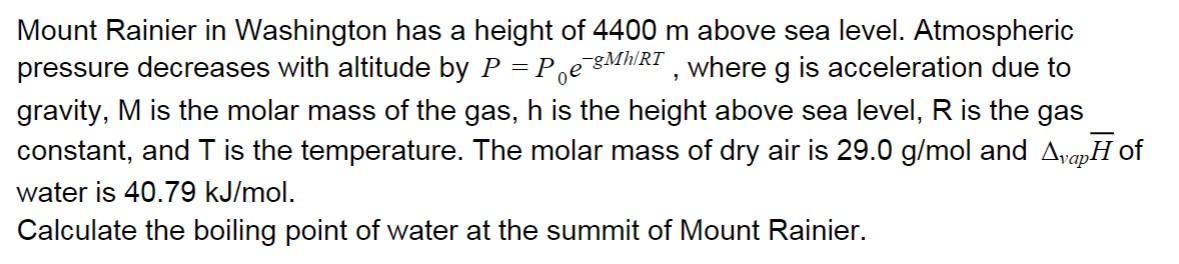
Mount Rainier in Washington has a height of 4400 m above sea level. Atmospheric pressure decreases with altitude by P = Pe 8MhRT , where g is acceleration due to gravity, M is the molar mass of the gas, h is the height above sea level, R is the gas constant, and T is the temperature. The molar mass of dry air is 29.0 g/mol and AvapH of water is 40.79 kJ/mol. Calculate the boiling point of water at the summit of Mount Rainier.
Step by Step Solution
3.46 Rating (146 Votes )
There are 3 Steps involved in it
Step: 1
the boiling poi...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Calculus Early Transcendentals
Authors: William L. Briggs, Lyle Cochran, Bernard Gillett
2nd edition
321954428, 321954424, 978-0321947345
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



