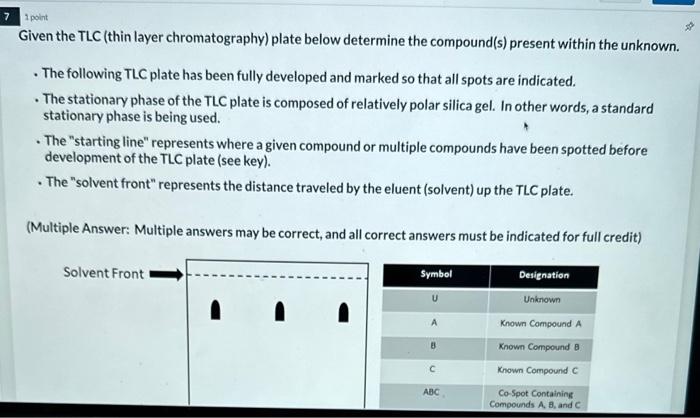
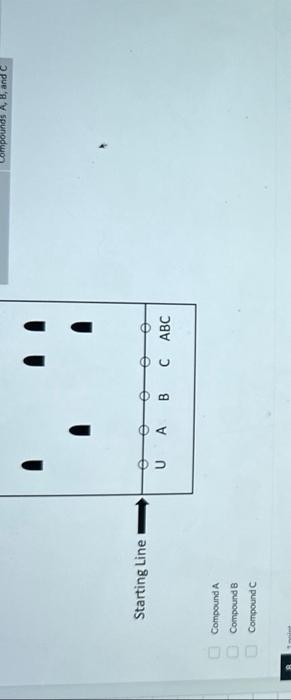
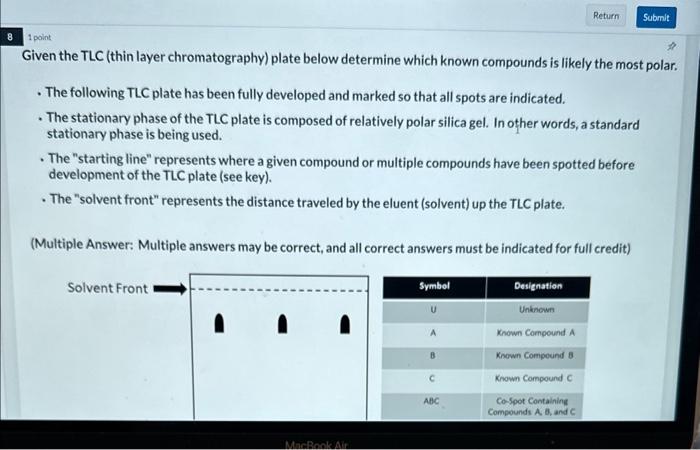
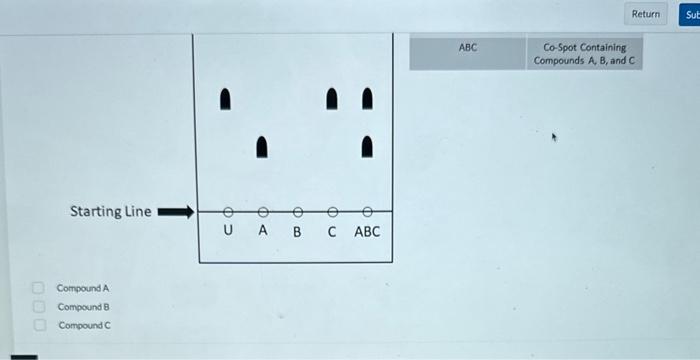
(Multiple Choice: Only one answer is correct, and only one answer can be selected) The solid phase is the stationary phase and the liquid phase is the mobile phase. The solid phase is the stationary phase and the gas phase is the mobile phase. The liquid phase is the stationary phase and the solid phase is the mobile phase. The liquid phase is the stationary phase and the gas phase is the mobile phase. The gas phase is the stationary phase and the solid phase is the mobile phase. The gas phase is the stationary phase and the liquid phase is the mobile phase. 1 point Which of the following chromatographic methods seperates components based on their boiling point, as well as their affinity for the stationary phase? (Multiple Choice: Only one answer is correct, and only one answer can be selected) High Performance Liquid Chromatography Thin Layer Chromatography Gas Chromatography 1 polint Which of the following chromatographic methods would allow one to collect the separated components? (Multiple Choice: Only one answer is correct, and only one answer can be selected) High Performance Liquid Chromatography Thin Layer Chromatography Gas Chromatography 1 poink Which of the following is true regarding thin layer chromatography (TLC)? (Multiple Choice: Only one answer is correct, and only one answer can be selected) The solid phase is the stationary phase and the Iiquid phase is the moblle phase. The solid phase is the stationary phase and the gas phase is the mobile phase. The liquid phase is the stationary phase and the solid phase is the mobile phase. The liquid phase is the stationary phase and the gas phase is the mobile phase. The gas phase is the stationary phuse and the solid phase is the mobile phase. The gas phase is the stationary phase and the liquid phase is the mobile phase. Given the TLC (thin layer chromatography) plate below determine the compound(s) present within the unknown. - The following TLC plate has been fully developed and marked so that all spots are indicated. - The stationary phase of the TLC plate is composed of relatively polar silica gel. In other words, a standard stationary phase is being used. - The "starting line" represents where a given compound or multiple compounds have been spotted before development of the TLC plate (see key). - The "solvent front" represents the distance traveled by the eluent (solvent) up the TLC plate. (Multiple Answer: Multiple answers may be correct, and all correct answers must be indicated for full credit) Starting Line Compound A Compound B Compound C Given the TLC (thin layer chromatography) plate below determine which known compounds is likely the most polar - The following TLC plate has been fully developed and marked so that all spots are indicated. - The stationary phase of the TLC plate is composed of relatively polar silica gel. In other words, a standard stationary phase is being used. - The "starting line" represents where a given compound or multiple compounds have been spotted before development of the TLC plate (see key). - The "solvent front" represents the distance traveled by the eluent (solvent) up the TLC plate. (Multiple Answer: Multiple answers may be correct, and all correct answers must be indicated for full credit) Compound A Compound 8 Compound C