Answered step by step
Verified Expert Solution
Question
1 Approved Answer
H20 N2 H25 CO2 C1 C2 C3 n-C4 n-CS C7+ 1. 30% 2. 5% 3. 5% MW (lbm/lb mole) 18.015 20.010 4.5% 34.080 44.010
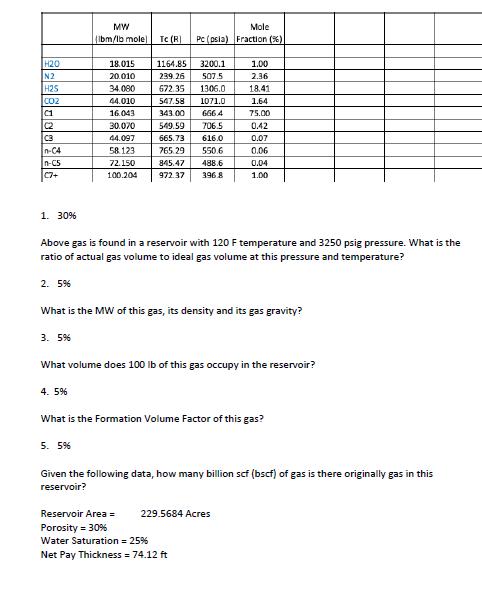
H20 N2 H25 CO2 C1 C2 C3 n-C4 n-CS C7+ 1. 30% 2. 5% 3. 5% MW (lbm/lb mole) 18.015 20.010 4.5% 34.080 44.010 16.043 30.070 44.097 58.123 72.150 100.204 Tc (R) 5. 5% Mole Pc (psia) Fraction (%) 1164.85 3200.1 239.26 507.5 672.35 1306.0 547.58 1071.0 343.00 666.4 549.59 706.5 665.73 616.0 765.29 550.6 845.47 488.6 972.37 396.8 Above gas is found in a reservoir with 120 F temperature and 3250 psig pressure. What is the ratio of actual gas volume to ideal gas volume at this pressure and temperature? == 1.00 2.36 18.41 What is the MW of this gas, its density and its gas gravity? 1.64 75.00 0.42 0.07 0.06 What volume does 100 lb of this gas occupy in the reservoir? Reservoir Area = Porosity = 30% Water Saturation = 25% Net Pay Thickness = 74.12 ft 229.5684 Acres 0.04 1.00 What is the Formation Volume Factor of this gas? Given the following data, how many billion scf (bscf) of gas is there originally gas in this reservoir?
Step by Step Solution
★★★★★
3.50 Rating (163 Votes )
There are 3 Steps involved in it
Step: 1
Solution Given Absolute Pab Pab Ratio ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


