Answered step by step
Verified Expert Solution
Question
1 Approved Answer
my first answer was 2.66 X 10^-6 and it was wrong. please provide the correct answer. Lab insirumenta have a linit of detection ithe smallst
my first answer was 2.66 X 10^-6 and it was wrong. please provide the correct answer. 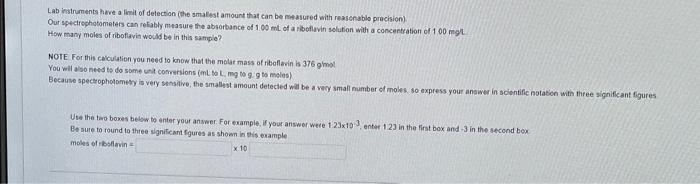
Lab insirumenta have a linit of detection ithe smallst amount that can be matesured with reassonsblo precision) Our iptcirephotomaters can retably measure the absorbance of 1.00 mt of a ribeflavin selution with a concentation of 100mgl How many moles of riboftavia would be in thas sample? NOIE For this calculation you need to know that the molar mass of riboflavin is 376.9mol You will also need ts do some unt cohrersions (inL bo L. mig to 9.9 to moles) Because speceophohomery is very shositive, the smallest amount detected will be a very small mumber of moles so express your answer in soientific notation with three significant figures Use the the boxes bebiw to enter your answer. For example, if your answor wece 1.23103, enter 1.23 in the first box and 3 in the stcend box. Be sute to round to thee signiseant fgures as shown in this example moles of rbsflavin = 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


