Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A. Solubility of Solids in Water and Cyclohexane Fill in the table with your solubility data (s soluble, i-insoluble, ss slightly soluble). Intermolecular Forces
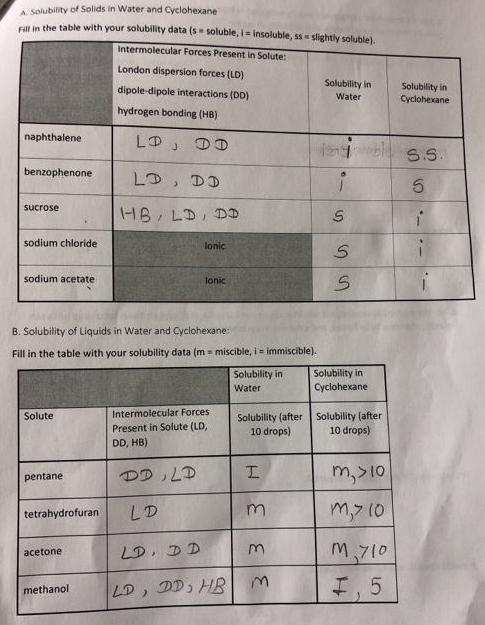


A. Solubility of Solids in Water and Cyclohexane Fill in the table with your solubility data (s soluble, i-insoluble, ss slightly soluble). Intermolecular Forces Present in Solute: naphthalene benzophenone sucrose sodium chloride sodium acetate Solute pentane tetrahydrofuran B. Solubility of Liquids in Water and Cyclohexane: Fill in the table with your solubility data (m= miscible, i = immiscible). acetone London dispersion forces (LD) dipole-dipole interactions (DD) hydrogen bonding (HB) DD methanol LD, DD HB, LD, DD lonic Tonic Intermolecular Forces Present in Solute (LD, DD, HB) DD, LD LD LD, DD LD, DD, HB Solubility in Water Solubility (after 10 drops) H 33 3 Solubility in Water S SS Solubility in Cyclohexane Solubility (after 10 drops) m, >10 m,> 10 M710 I. 5 Solubility in Cyclohexane S.S. 5 4. For the molecular compounds you investigated in Parts A and B, explain the relationship between polarity, intermolecular forces, and solubility in water. Provide specific examples from part A: Provide specific examples from part B: 5. For the molecular compounds you investigated in Parts A and B, explain the relationship between polarity, intermolecular forces, and solubility in cyclohexane. Provide specific examples from part A: Provide specific examples from part B: 10 6. Are the ionic compounds you investigated in Part A more soluble in water or cyclohexane? What is the reason for this observed behavior?
Step by Step Solution
★★★★★
3.43 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
ATTACHED IMAGE Answer 4 As the polarity of the solvent increases its solubility also increases Polar ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


