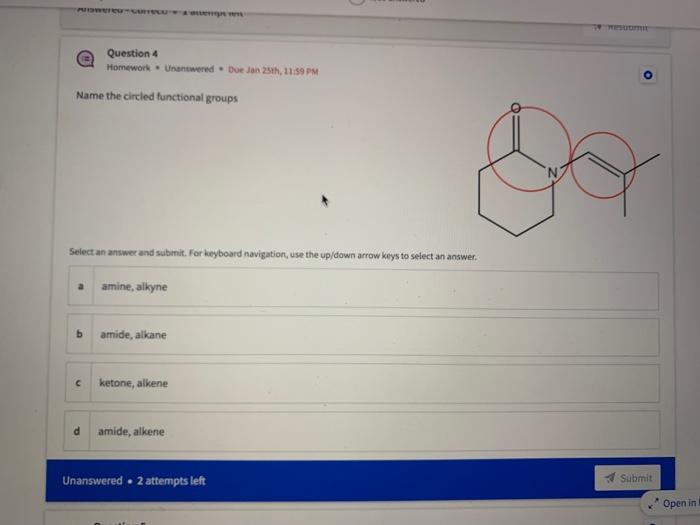
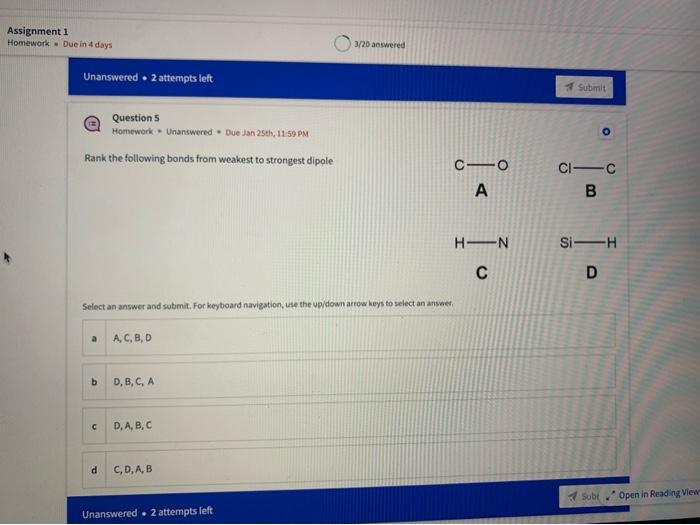
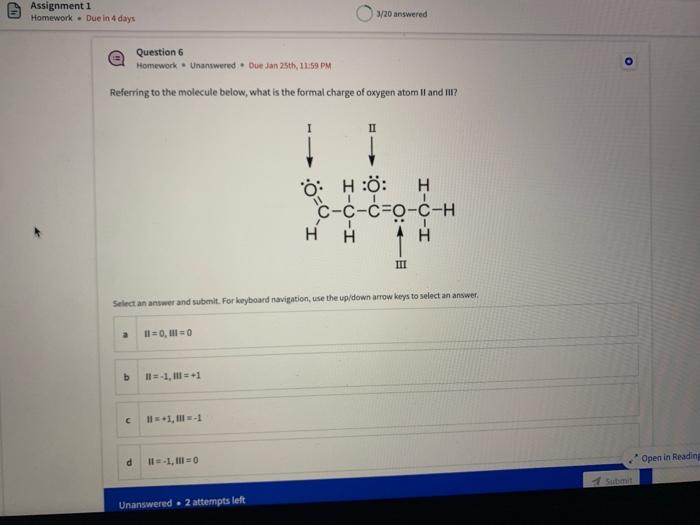
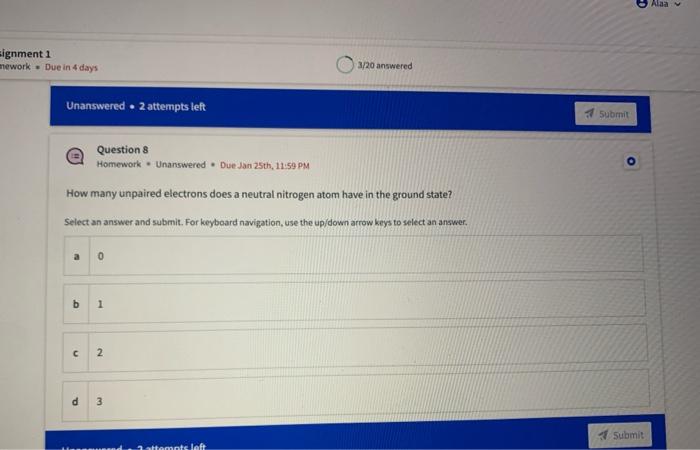
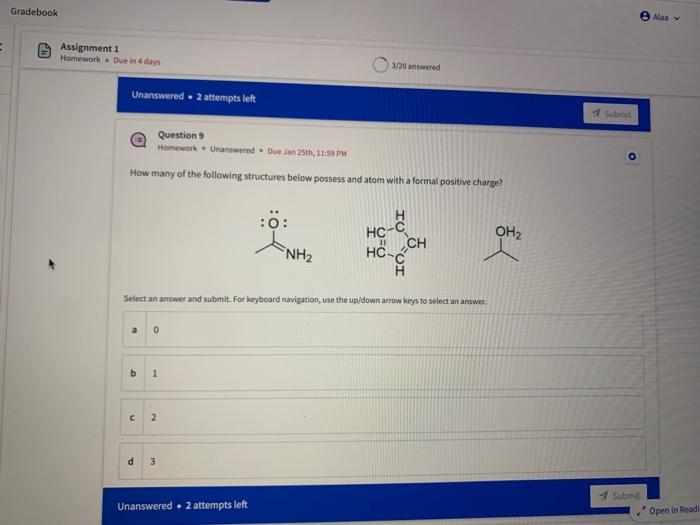
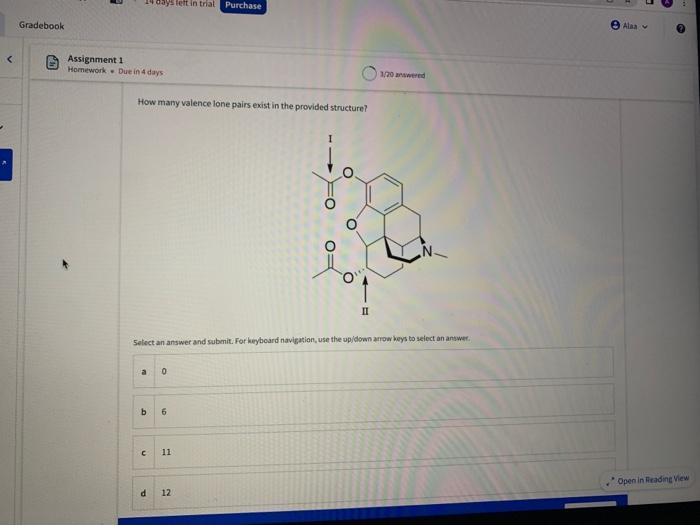
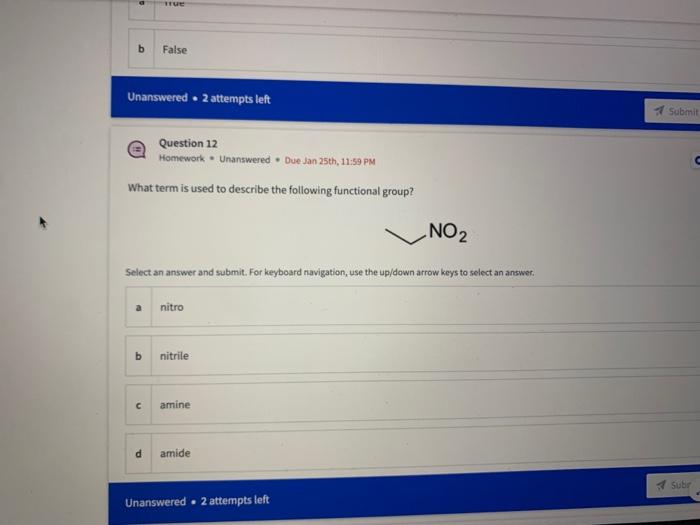
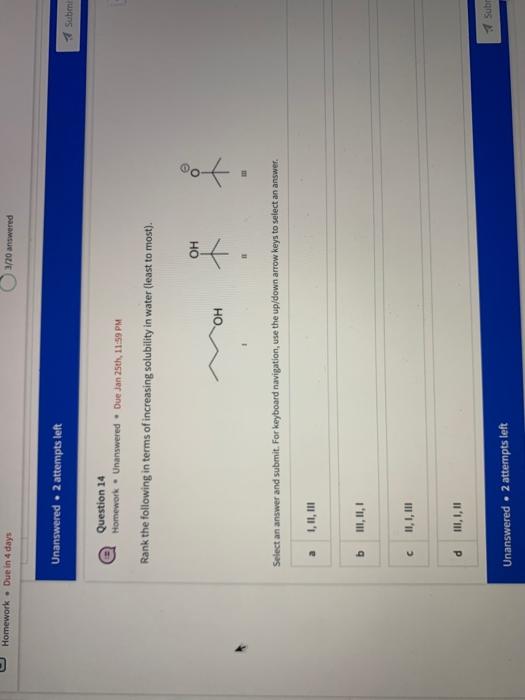
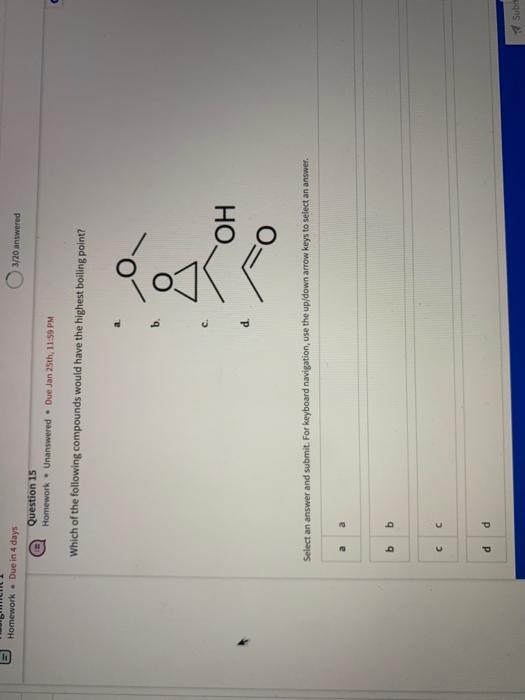
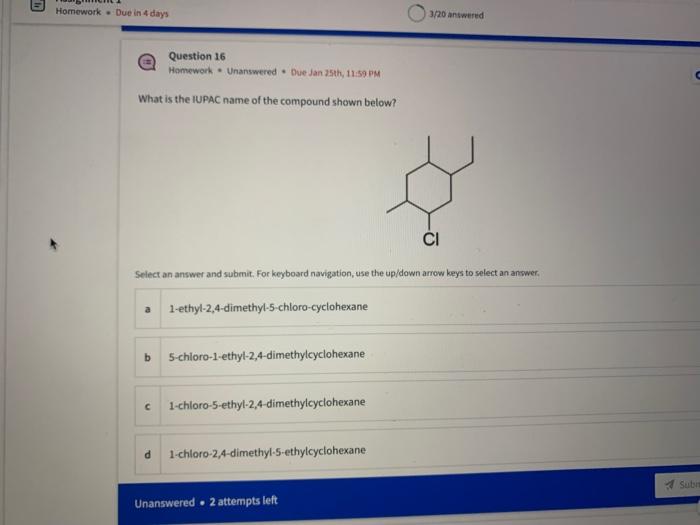
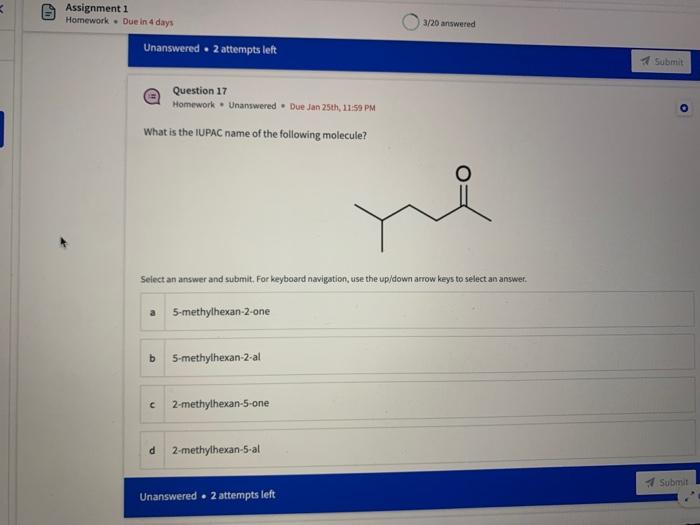
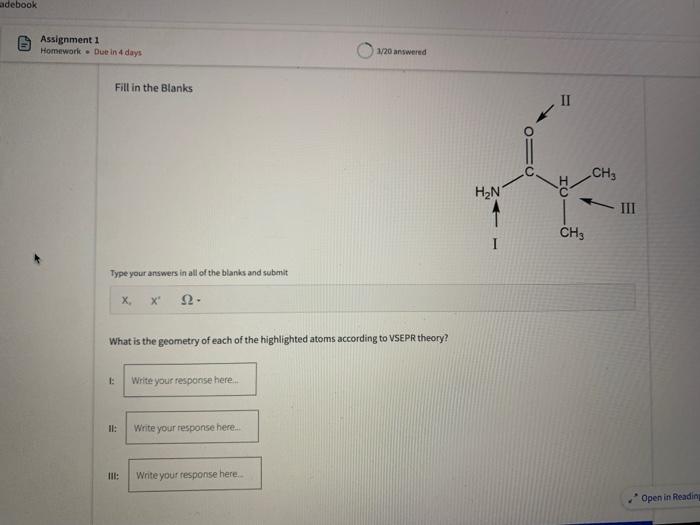
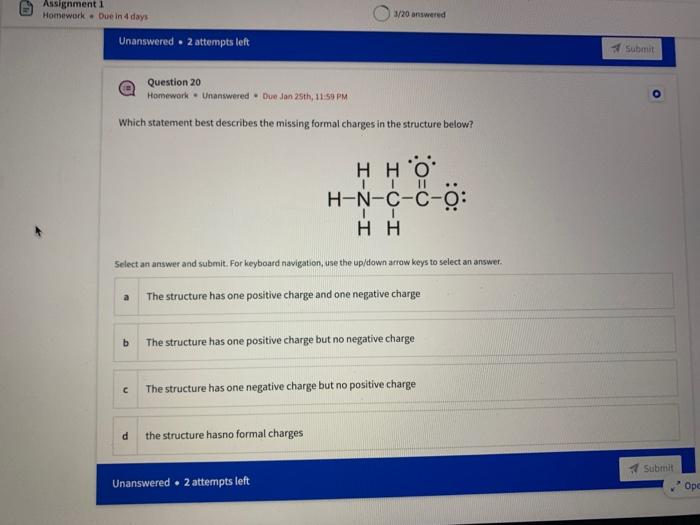
Name the circled functional groups Rank the following bonds from weakest to strongest dipole ClC A 8 SiH Select an answer and submit, For keyboard navigation, use the upidown arcow keys to select an answer, a A,C,B,D b D, B, C, A c D,A,B,C d C,D,A,B Referring to the molecule below, what is the formal charge of oxygen atom II and II? Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a II=0,III=0 II=1,II=+1 II=+1,III=1 II=1,III=0 Which of the following represents the ground state electron configuration for a O2 - anion? Select an answer and submit. For keyboord navigation, use the op (down acrow kryin to select an acswer. 1s22s22p4 b 1s22s22p2 1s22s22p6 d 2s22p4 Question 8 Homework - Unanswered - Due Jan 25th, 11:59 PM How many unpaired electrons does a neutral nitrogen atom have in the ground state? Select an answer and submit. For keybeard navigation, use the up/down arrow keys to select an answer. a 0 b 1 c 2 d 3 How many of the following structures below possess and atom with a formal positive charge? Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an arswer: How many valence lone pairs exist in the provided structure? Select an answer and submit: For kerboard navigation, use the op/down arrow heys to seiect an answer. Homework - Unanswered - Due Jan 25th, 11:59 PM In a OH bond, the electron density is skewed towards the hydrogen atom. Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a True b False What term is used to describe the following functional group? NO2 Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a nitro b nitrile c amine Unanswered 2 attempts left 3/20 answered Question 13 Homework - Unanswered - Due Jan 25th, 11-59 PM Which of the following represents the strongest intermolecular force? Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answef. a London dispersion b electrostatic (ion-ion) c dipole-dipole d hydrogen bonding Unanswered 2 attempts left Question 14 Homework - Unanswered * Due Jan 25 th, 11 -59 PM Rank the following in terms of increasing solubility in water (least to most). Question 14 Homework - Unanswered - Due Jan 25th, 11:59 PM Rank the following in terms of increasing solubility in water (least to most). Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. Homework - Due in 4 days 3/20 answered Question 15 Homework - Unanswered * Due Jan 25th, 11ts9 PM Which of the following compounds would have the highest boiling point? a. b. C. d. Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. b b c c d d Question 16 Homework - Unanswered * Due Jan 25th, 11.59 pM What is the IUPAC name of the compound shown below? Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a 1-ethyl-2,4-dimethyl-5-chloro-cyclohexane b 5-chloro-1-ethyl-2,4-dimethylcyclohexane c 1-chloro-5-ethyl-2,4-dimethylcyclohexane 3/20 answered Unanswered - 2 attempts left Question 17 Homework - Unanswered - Due Jan 25th, 11:59 PM What is the IUPAC name of the following molecule? Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a 5-methylhexan-2-one b 5 -methylhexan-2-al c 2 -methylhexan-5-one d 2-methylhexan-5-al In general, the stronger the intermolecular forces are between molecules, the higher the melting point will be. Select an answer and submit. For keyboard navigation, use the up/down arrow heys to select an answer. a True b False Fill in the Blanks Type your arswers in all of the blanks and submlt What is the geometry of each of the highlighted atoms according to VSEPR theory? Which statement best describes the missing formal charges in the structure below? Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a The structure has one positive charge and one negative charge b The structure has one positive charge but no negative charge c The structure has one negative charge but no positive charge d the structure hasno formal charges