Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Need answers for part B HW Ch 14 Begin Date: 4/8/2024 12:01:00 AM -- Due Date: 4/14/2024 11:55:00 PM End Date: 4/15/2024 11:55:00 PM (13%)
Need answers for part B
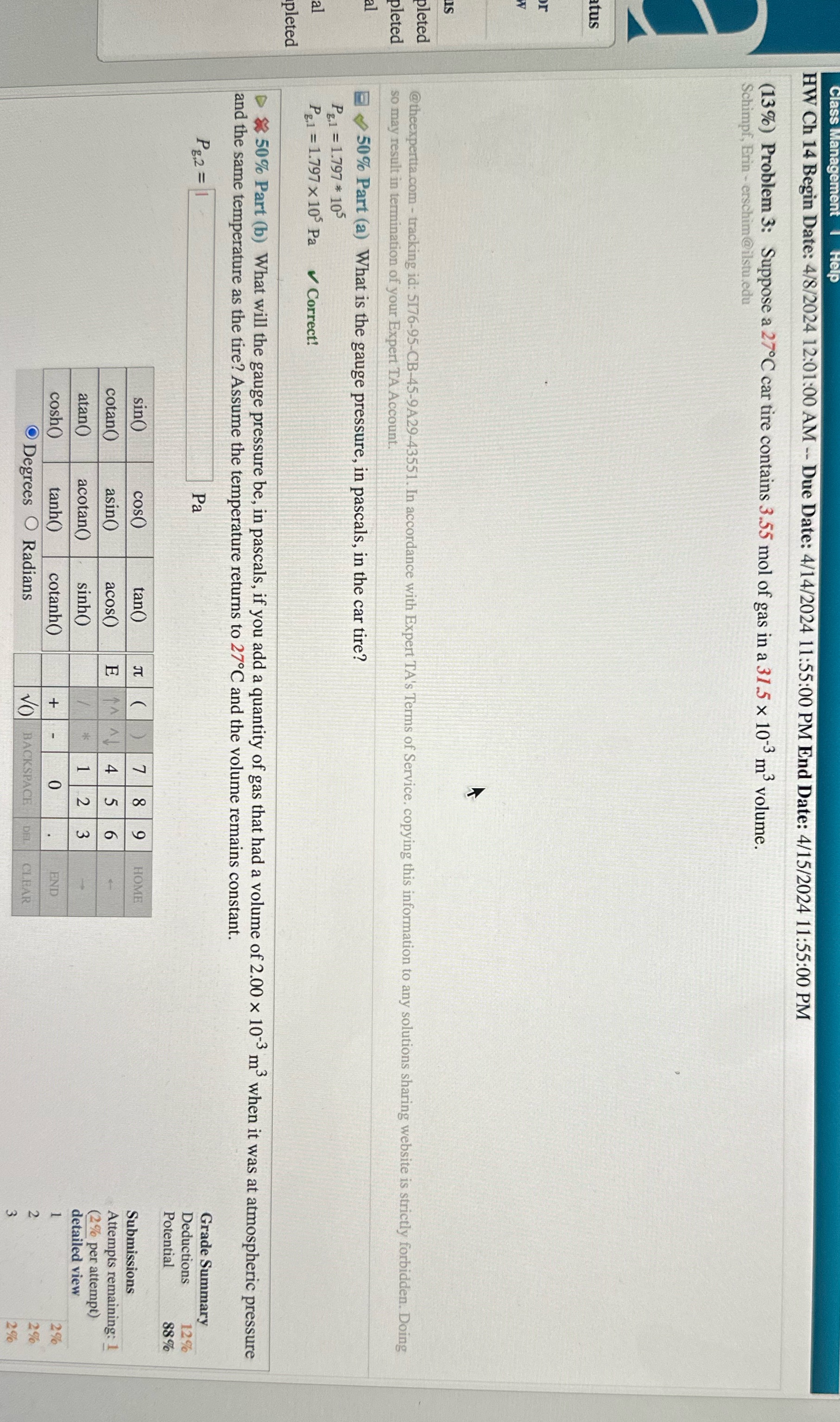 HW Ch 14 Begin Date: 4/8/2024 12:01:00 AM -- Due Date: 4/14/2024 11:55:00 PM End Date: 4/15/2024 11:55:00 PM (13%) Problem 3: Suppose a 27.C car tire contains 3.55 mol of gas in a 31.5 x 10 3 m volume. Schimpf, Erin - erschim @ilstu.edu us leted @theexpertta.com - tracking id: 5176-95-CB-45-9A29-43551. In accordance with Expert TA's Terms of Service. copying this information to any solutions sharing website is strictly forbidden. Doing pleted so may result in termination of your Expert TA Account. 50% Part (a) What is the gauge pressure, in pascals, in the car tire? Pg.1 = 1.797 * 105 Pg,1 = 1.797 x 105 Pa Correct! pleted *50% Part (b) What will the gauge pressure be, in pascals, if you add a quantity of gas that had a volume of 2.00 x 103 m' when it was at atmospheric pressure and the same temperature as the tire? Assume the temperature returns to 27C and the volume remains constant. Pg.2 = 1 Pa Grade Summary Deductions 12% Potential 88% sin() cos tan() OC O HOME Submissions cotan() asin( acos() Attempts remaining: 1 (2% per attempt) atan() acotan() sinh( N detailed view cosho tanh() cotanh() + END Degrees O Radians BACKSPACE DEL CLEAR
HW Ch 14 Begin Date: 4/8/2024 12:01:00 AM -- Due Date: 4/14/2024 11:55:00 PM End Date: 4/15/2024 11:55:00 PM (13%) Problem 3: Suppose a 27.C car tire contains 3.55 mol of gas in a 31.5 x 10 3 m volume. Schimpf, Erin - erschim @ilstu.edu us leted @theexpertta.com - tracking id: 5176-95-CB-45-9A29-43551. In accordance with Expert TA's Terms of Service. copying this information to any solutions sharing website is strictly forbidden. Doing pleted so may result in termination of your Expert TA Account. 50% Part (a) What is the gauge pressure, in pascals, in the car tire? Pg.1 = 1.797 * 105 Pg,1 = 1.797 x 105 Pa Correct! pleted *50% Part (b) What will the gauge pressure be, in pascals, if you add a quantity of gas that had a volume of 2.00 x 103 m' when it was at atmospheric pressure and the same temperature as the tire? Assume the temperature returns to 27C and the volume remains constant. Pg.2 = 1 Pa Grade Summary Deductions 12% Potential 88% sin() cos tan() OC O HOME Submissions cotan() asin( acos() Attempts remaining: 1 (2% per attempt) atan() acotan() sinh( N detailed view cosho tanh() cotanh() + END Degrees O Radians BACKSPACE DEL CLEAR Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


