Answered step by step
Verified Expert Solution
Question
1 Approved Answer
need answers now please 1 pts Question 4 You are massing out sodium bicarbonate powder for an experiment on acids and bases. First, you find
need answers now please 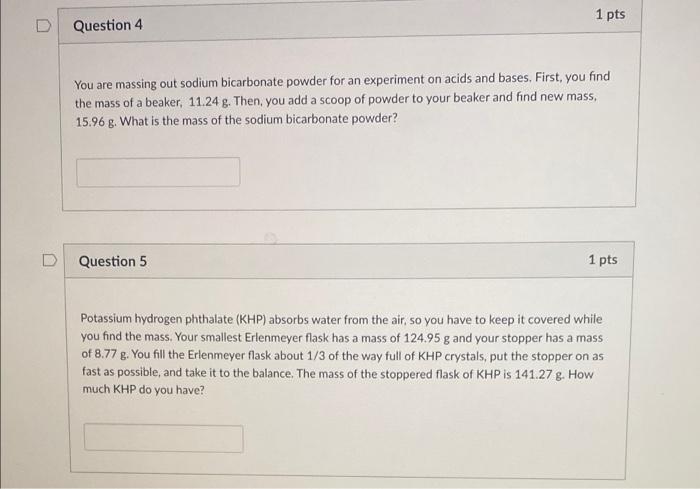
1 pts Question 4 You are massing out sodium bicarbonate powder for an experiment on acids and bases. First, you find the mass of a beaker, 11.24 g. Then, you add a scoop of powder to your beaker and find new mass, 15.96 g. What is the mass of the sodium bicarbonate powder? Question 5 1 pts Potassium hydrogen phthalate (KHP) absorbs water from the air, so you have to keep it covered while you find the mass. Your smallest Erlenmeyer flask has a mass of 124.95 g and your stopper has a mass of 8.77 g. You fill the Erlenmeyer flask about 1/3 of the way full of KHP crystals, put the stopper on as fast as possible, and take it to the balance. The mass of the stoppered flask of KHP is 141.27 8. How much KHP do you have 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


