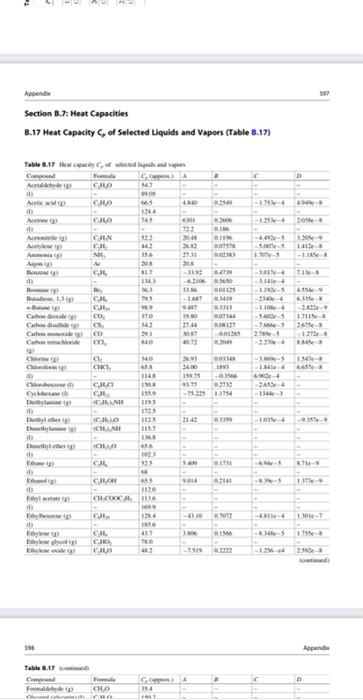
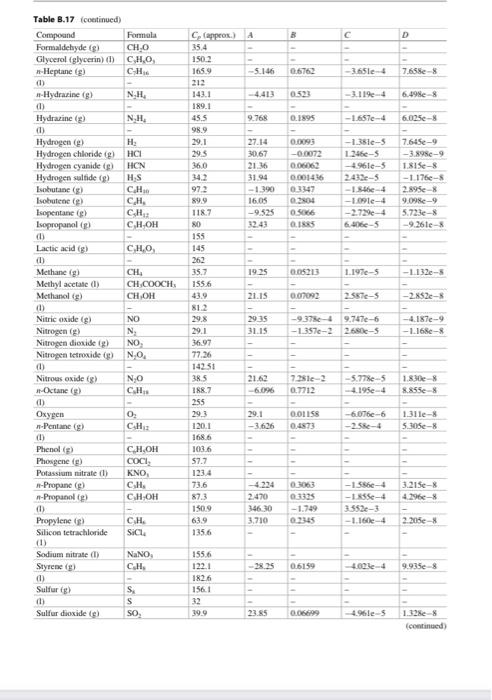
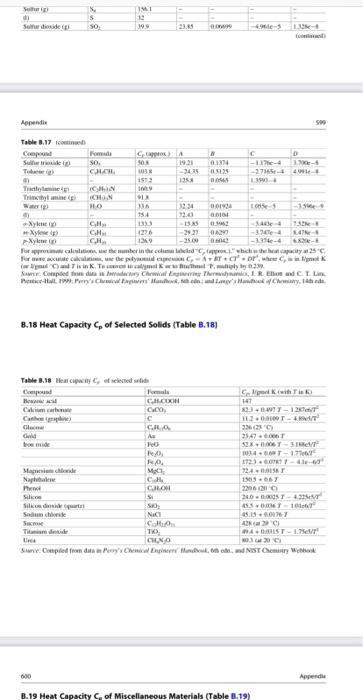
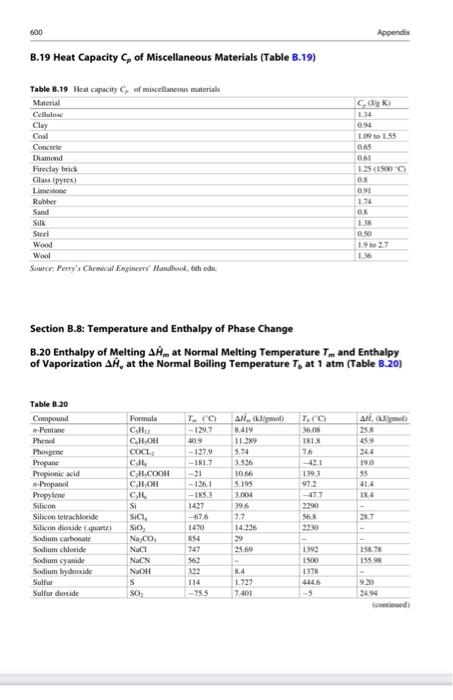
Nitric acid can be produced from the reaction of ammonia with oxygen according to the equation: 4 NH3 (9) + 502 (9) 4 NO(g) + 6H20 (9) 1 mol/min of ammonia and 7 molmin of air (79% N2, 21% O2) enters the roactor at 70 C. 75% conversion of ammonia is achieved inside the reactor and no side reactions occur. Calculate the heat requirement if the temperature of the gas leaving the reactor is 500 C. TE Appende Section B.J: Heat Capacities 8.17 Heat Capacity of selected Liquids and Vapors (Table 3.17) Table 1.17 Wow and Camel C. A CAO 1147 CM 40 -11- Aw 143 ANI 733 04 CHN 45 A A A TES CH 00338 II AL 356 17031 - Y th CJL TE TINS 4 A. LUM --- 24 33 LUS M. CJL CL CD C. 111 BOTH 0 14: 1344 -50-51711-5 S24- 2. -122 C D MO CKT -1 - M. 24 11 1.54e- 663 CL CE - PE -15 C De CH Is CO 1725 1118 BIT Duter tum 2141 10. 159 INTRO Duter REIS CAL CH 014 6 1 CHLO 1114 CJE 10 19 0 im C CO GLO EXT 1319 165 opende Table 3.17 Com Furma CHO hu 35.4 D 0.6762 -3.651e 7.6588 0523 - 3.114 6.4988 0.1895 -1.657e-4 6.025e-8 Table 8.17 continued) Compound Formula Formaldehyde (8) CHO Glycerol (glycerin) d) CHO Heptane (g) CH (1) -Hydrazine (8) NH (1) Hydrazine (g) NH (1) Hydrogen H Hydrogen chloride (g) HCI Hydrogen cyanide () HCN Hydrogen sulfide (8) HS Isobutane (g) C.H. Isobutene (g) CH Isopentane () CH Isopropanol C,H,OH (0 Lactic acid (3) CHO (1) Methane g CH Methyl acetate (1) CH.COOCH Methanol (g) CHOH (D) Nitric Oxide (g) NO Nitrogen (g) N Nitrogen dioxide) NO Nitrogen teroxide (8) NO (1) Nitrous oxide (8) NO -Octane (g) C.H. 0.0093 -0.0072 00862 0.001436 03147 304 05066 185 -1.351-5 12464-5 -4961-5 2433-5 -1.56-4 -ple- -2.729-4 6.06-5 7.645e-9 -3.8980-9 1.815-8 - 1.176- 2.895- 9.09- 5.723-8 -9.26e- $9.9 0.05213 1.197e-5 -1132-5 C Capprox.) A 35.4 150-2 1659 -5.146 212 1431 189.1 45.5 9.768 98.9 29.1 27.14 29.5 30,67 36.0 21.16 31.94 972 -1.390 16.05 1187 -9.525 20 155 145 262 35.7 1925 1556 43.9 21.15 812 29.8 2915 29.1 31.15 36.97 77.26 14231 38.5 21.62 1887 -6.096 255 29.1 120.1 -3.626 168.6 103.6 57.7 1234 73.6 87.3 2.470 1509 346 30 63.9 3.710 1356 0.07092 2581-5 -2.2- -9.374 -1357e-2 9.7474-6 2680-5 -4.187-9 -1.168-8 73310-3 0.7712 -5.778-5 -4.195e-4 1.830-8 8.855e-8 0 CH 2 0.01158 0.4873 -6.076-6 -2.586-4 1.311-3 5.305- CHOH COCI, KNO, C.H. 3.215e-8 4.1966-8 C.HOH Oxygen -Pentane (8) (1) Phenol (g) Phosgene (g) Potassium nitrate (1) -Propune () -Propanol) (1) Propylene Silicon tetrachloride (1) Sodium nitrate (1) Styreme (g) (1) Sulfuri) d) Sulfur dioxide s) 03063 0.1325 -1.749 0.2345 -1.566-4 -Se-4 3.552e-3 -1.16-4 2.205 CH. SICH NaNO . 38.25 0.6159 4.623-1 9935e-8 155.6 122.1 182.6 156.1 32 39.9 S SO 23.85 0.06.10 4.96e-5 1.3288 (continued) 41 19 19 5 SO Side 23.05 -5 1. Table 3.17 Cep! From A D Suite SO 08 01114 - - -275-4. 1513 10 Time CHIN CHON 9 HE 16 3334 10193 -1 - 514 20 Xy CH SS 3. Xyles CH 1276 -2921 DA --- Nyer CH 12 -25 -1994-4-2 Forecast, the user inte med tich the capacity 25C For we care te py-or'where iwliin Talway2 Completo di www.Eland CTL Pestically we don't way. The 8.18 Heat Capacity of selected Solids (Table 1.18) Table 8.18 Heuwe collected Compound Clikih in CRO HT Calcium carente CO 33+ WT-12 c 102.00000 G C. 326239 Gid A 23,47 SEXT- 103-17 1T2) Sanidade M 724 CH 2013.06 Phenol CLOM 2200 Sice su 2.43 silitas SO 850101 Seche NO 3.13.00 Secm CA LORE Time TK ISTIT No Sune Compiled from date Changer und NIST Chemistry Web 600 B. 19 Heat Capacity of Miscellaneous Materials (Table 3.19) 600 Appendios B.19 Heat Capacity of Miscellaneous Materials (Table B.19) C.dk 1.34 Table 8.19 Heat capacity of miscellaneous material Material Cellulo Clay Coal Concrete Diamond Fireclay brick Glass pyrex Limestone Rubber Sund Silk Steel Wood Wool Source: Perry's Chemical Engineers' Handhat, then. 1.09 1.55 0.65 0.61 1.25(1900 OX 091 1.74 OS 1.35 040 1922 1. Section 3.8: Temperature and Enthalpy of Phase Change B.20 Enthalpy of Melting Amat Normal Melting Temperature T.. and Enthalpy of Vaporization AH, at the Normal Boiling Temperature T, at 1 atm (Table B.20) AN 244 190 35 Table 3.20 Compound --Pentane Phone Phasene Propane Progimni acid -Propanol Propylene Silicon Silicon tetrachloride Silicon diside quatt) Sodium carbonate Sodium chloride Sodium syanide Sodium hydroxide Sulfur Sulfur dioxide T. -129.7 409 -- 127.9 -1817 -21 126.1 -IR 1427 -676 1470 854 747 Formula CH C.H.ON COXCL CH CH.COOH CH.CH CH Si SIL SIO Na.co. Naci NaCN NAOH s SO a klimal) 8.419 11.289 5.74 1526 10.66 5.195 3.004 396 7.7 14.226 29 25.00 TAO 16.0N INEX 76 -42.1 1393 972 -477 2290 414 1 S6 2230 1392 1500 15. 15 322 114 -755 84 1.727 7401 4446 9 2494 Nitric acid can be produced from the reaction of ammonia with oxygen according to the equation: 4 NH3 (9) + 502 (9) 4 NO(g) + 6H20 (9) 1 mol/min of ammonia and 7 molmin of air (79% N2, 21% O2) enters the roactor at 70 C. 75% conversion of ammonia is achieved inside the reactor and no side reactions occur. Calculate the heat requirement if the temperature of the gas leaving the reactor is 500 C. TE Appende Section B.J: Heat Capacities 8.17 Heat Capacity of selected Liquids and Vapors (Table 3.17) Table 1.17 Wow and Camel C. A CAO 1147 CM 40 -11- Aw 143 ANI 733 04 CHN 45 A A A TES CH 00338 II AL 356 17031 - Y th CJL TE TINS 4 A. LUM --- 24 33 LUS M. CJL CL CD C. 111 BOTH 0 14: 1344 -50-51711-5 S24- 2. -122 C D MO CKT -1 - M. 24 11 1.54e- 663 CL CE - PE -15 C De CH Is CO 1725 1118 BIT Duter tum 2141 10. 159 INTRO Duter REIS CAL CH 014 6 1 CHLO 1114 CJE 10 19 0 im C CO GLO EXT 1319 165 opende Table 3.17 Com Furma CHO hu 35.4 D 0.6762 -3.651e 7.6588 0523 - 3.114 6.4988 0.1895 -1.657e-4 6.025e-8 Table 8.17 continued) Compound Formula Formaldehyde (8) CHO Glycerol (glycerin) d) CHO Heptane (g) CH (1) -Hydrazine (8) NH (1) Hydrazine (g) NH (1) Hydrogen H Hydrogen chloride (g) HCI Hydrogen cyanide () HCN Hydrogen sulfide (8) HS Isobutane (g) C.H. Isobutene (g) CH Isopentane () CH Isopropanol C,H,OH (0 Lactic acid (3) CHO (1) Methane g CH Methyl acetate (1) CH.COOCH Methanol (g) CHOH (D) Nitric Oxide (g) NO Nitrogen (g) N Nitrogen dioxide) NO Nitrogen teroxide (8) NO (1) Nitrous oxide (8) NO -Octane (g) C.H. 0.0093 -0.0072 00862 0.001436 03147 304 05066 185 -1.351-5 12464-5 -4961-5 2433-5 -1.56-4 -ple- -2.729-4 6.06-5 7.645e-9 -3.8980-9 1.815-8 - 1.176- 2.895- 9.09- 5.723-8 -9.26e- $9.9 0.05213 1.197e-5 -1132-5 C Capprox.) A 35.4 150-2 1659 -5.146 212 1431 189.1 45.5 9.768 98.9 29.1 27.14 29.5 30,67 36.0 21.16 31.94 972 -1.390 16.05 1187 -9.525 20 155 145 262 35.7 1925 1556 43.9 21.15 812 29.8 2915 29.1 31.15 36.97 77.26 14231 38.5 21.62 1887 -6.096 255 29.1 120.1 -3.626 168.6 103.6 57.7 1234 73.6 87.3 2.470 1509 346 30 63.9 3.710 1356 0.07092 2581-5 -2.2- -9.374 -1357e-2 9.7474-6 2680-5 -4.187-9 -1.168-8 73310-3 0.7712 -5.778-5 -4.195e-4 1.830-8 8.855e-8 0 CH 2 0.01158 0.4873 -6.076-6 -2.586-4 1.311-3 5.305- CHOH COCI, KNO, C.H. 3.215e-8 4.1966-8 C.HOH Oxygen -Pentane (8) (1) Phenol (g) Phosgene (g) Potassium nitrate (1) -Propune () -Propanol) (1) Propylene Silicon tetrachloride (1) Sodium nitrate (1) Styreme (g) (1) Sulfuri) d) Sulfur dioxide s) 03063 0.1325 -1.749 0.2345 -1.566-4 -Se-4 3.552e-3 -1.16-4 2.205 CH. SICH NaNO . 38.25 0.6159 4.623-1 9935e-8 155.6 122.1 182.6 156.1 32 39.9 S SO 23.85 0.06.10 4.96e-5 1.3288 (continued) 41 19 19 5 SO Side 23.05 -5 1. Table 3.17 Cep! From A D Suite SO 08 01114 - - -275-4. 1513 10 Time CHIN CHON 9 HE 16 3334 10193 -1 - 514 20 Xy CH SS 3. Xyles CH 1276 -2921 DA --- Nyer CH 12 -25 -1994-4-2 Forecast, the user inte med tich the capacity 25C For we care te py-or'where iwliin Talway2 Completo di www.Eland CTL Pestically we don't way. The 8.18 Heat Capacity of selected Solids (Table 1.18) Table 8.18 Heuwe collected Compound Clikih in CRO HT Calcium carente CO 33+ WT-12 c 102.00000 G C. 326239 Gid A 23,47 SEXT- 103-17 1T2) Sanidade M 724 CH 2013.06 Phenol CLOM 2200 Sice su 2.43 silitas SO 850101 Seche NO 3.13.00 Secm CA LORE Time TK ISTIT No Sune Compiled from date Changer und NIST Chemistry Web 600 B. 19 Heat Capacity of Miscellaneous Materials (Table 3.19) 600 Appendios B.19 Heat Capacity of Miscellaneous Materials (Table B.19) C.dk 1.34 Table 8.19 Heat capacity of miscellaneous material Material Cellulo Clay Coal Concrete Diamond Fireclay brick Glass pyrex Limestone Rubber Sund Silk Steel Wood Wool Source: Perry's Chemical Engineers' Handhat, then. 1.09 1.55 0.65 0.61 1.25(1900 OX 091 1.74 OS 1.35 040 1922 1. Section 3.8: Temperature and Enthalpy of Phase Change B.20 Enthalpy of Melting Amat Normal Melting Temperature T.. and Enthalpy of Vaporization AH, at the Normal Boiling Temperature T, at 1 atm (Table B.20) AN 244 190 35 Table 3.20 Compound --Pentane Phone Phasene Propane Progimni acid -Propanol Propylene Silicon Silicon tetrachloride Silicon diside quatt) Sodium carbonate Sodium chloride Sodium syanide Sodium hydroxide Sulfur Sulfur dioxide T. -129.7 409 -- 127.9 -1817 -21 126.1 -IR 1427 -676 1470 854 747 Formula CH C.H.ON COXCL CH CH.COOH CH.CH CH Si SIL SIO Na.co. Naci NaCN NAOH s SO a klimal) 8.419 11.289 5.74 1526 10.66 5.195 3.004 396 7.7 14.226 29 25.00 TAO 16.0N INEX 76 -42.1 1393 972 -477 2290 414 1 S6 2230 1392 1500 15. 15 322 114 -755 84 1.727 7401 4446 9 2494