Answered step by step
Verified Expert Solution
Question
1 Approved Answer
need help answering 3 3. Explain why the experimentally determined values for the specific rotation vary from literature values, if necessary. Conclusions: The relationship between
need help answering 3 
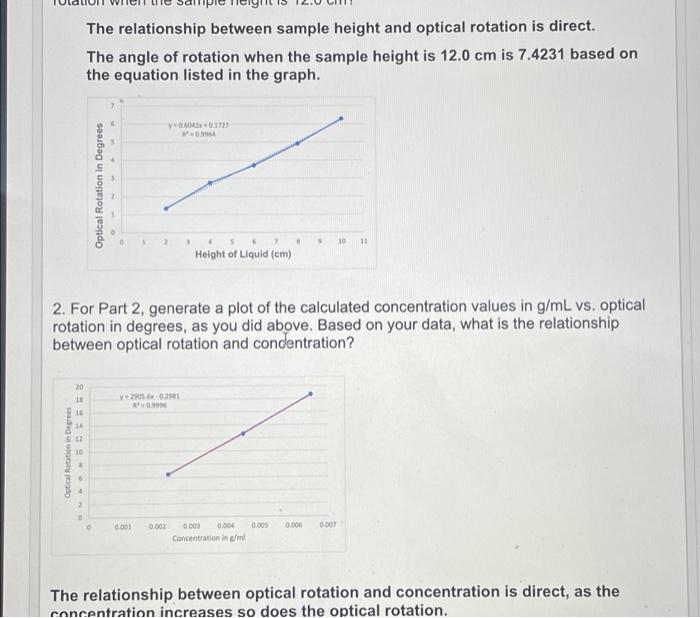
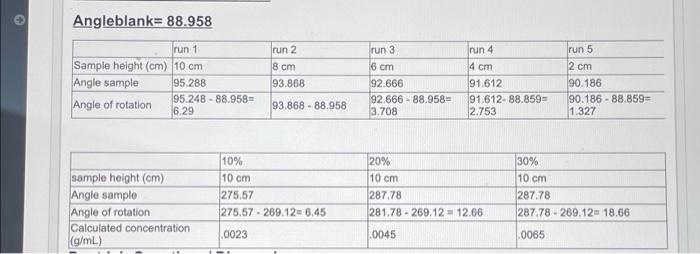
3. Explain why the experimentally determined values for the specific rotation vary from literature values, if necessary. Conclusions: The relationship between sample height and optical rotation is direct. The angle of rotation when the sample height is 12.0 cm is 7.4231 based on the equation listed in the graph. v0.1721 Optical Rotation in Degrees 7 10 11 Height of Liquid (cm) 2. For Part 2, generate a plot of the calculated concentration values in g/mL vs. optical rotation in degrees, as you did above. Based on your data, what is the relationship between optical rotation and condentration? 20 1 2002901 0.999 16 14 Optical Rotation in Dept 0.001 0.003 0.005 0.000 DO 0.003 0.004 Concentration in /ml The relationship between optical rotation and concentration is direct, as the concentration increases so does the optical rotation. Angleblank= 88.958 run 1 Sample height (cm) 10 cm Angle sample 95.288 Angle of rotation 95.248 - 88.958 6.29 run 2 8 cm 93.868 run 3 6 cm 92.666 92.666 - 88.958- 3.708 run 4 4 cm 91.612 91.612- 88.859 2.753 run 5 2 cm 90.186 90.186 - 88.859- 1.327 93.868 - 88.958 sample height (cm) Angle sample Angle of rotation Calculated concentration (g/mL) 10% 10 cm 275.57 275.57-269.12 6.45 20% 10 cm 287.78 281.78 - 269.12 - 12.66 30% 10 cm 287.78 287 78 - 269.12. 18.66 .0023 0045 0065 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


