Answered step by step
Verified Expert Solution
Question
1 Approved Answer
need help asap Hydrogen is produced in the steam reforming of propane: CH3(g) + 3H2O(v) - 3CO(g) + 7H2(g) - The water-gas shift reaction also
need help asap 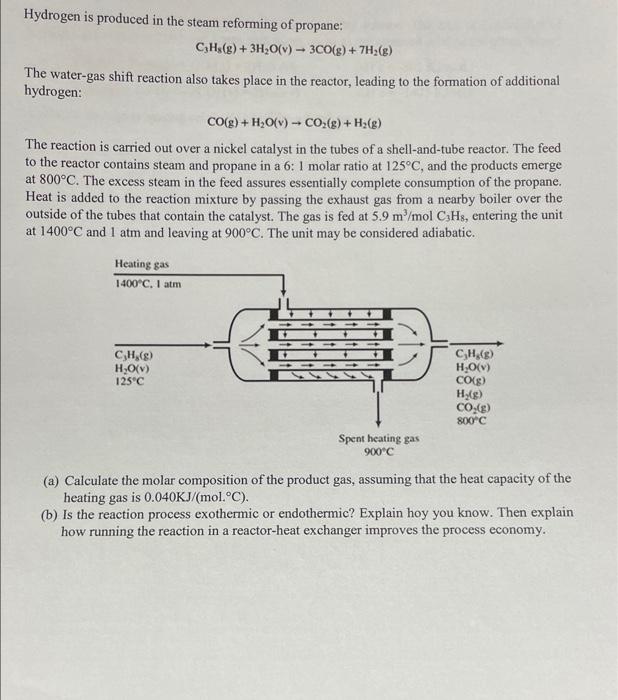
Hydrogen is produced in the steam reforming of propane: CH3(g) + 3H2O(v) - 3CO(g) + 7H2(g) - The water-gas shift reaction also takes place in the reactor, leading to the formation of additional hydrogen: CO(g) + H2O(v) - CO2(g) + H2(g) The reaction is carried out over a nickel catalyst in the tubes of a shell-and-tube reactor. The feed to the reactor contains steam and propane in a 6:1 molar ratio at 125C, and the products emerge at 800C. The excess steam in the feed assures essentially complete consumption of the propane. Heat is added to the reaction mixture by passing the exhaust gas from a nearby boiler over the outside of the tubes that contain the catalyst. The gas is fed at 5.9 m/mol CHs, entering the unit at 1400C and 1 atm and leaving at 900C. The unit may be considered adiabatic. Heating gas 1400C. I am CH,(8) H2O(v) 125C C,H,(8) H2O(V) CO(S) H2(g) CO2(g) 800C Spent heating gas 900C (a) Calculate the molar composition of the product gas, assuming that the heat capacity of the heating gas is 0.040KJ/(mol.C). (b) Is the reaction process exothermic or endothermic? Explain hoy you know. Then explain how running the reaction in a reactor-heat exchanger improves the process economy 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


