Answered step by step
Verified Expert Solution
Question
1 Approved Answer
need help on ALL please 4. Bacteria can sour wine and beer by converting ethanol (C2H5OH) into ethanoic ac (CH3COOH) : Overall Equation: C2H5OH(1)+O2(g)CH3COOH(1)+H2O(1) The
need help on ALL please 
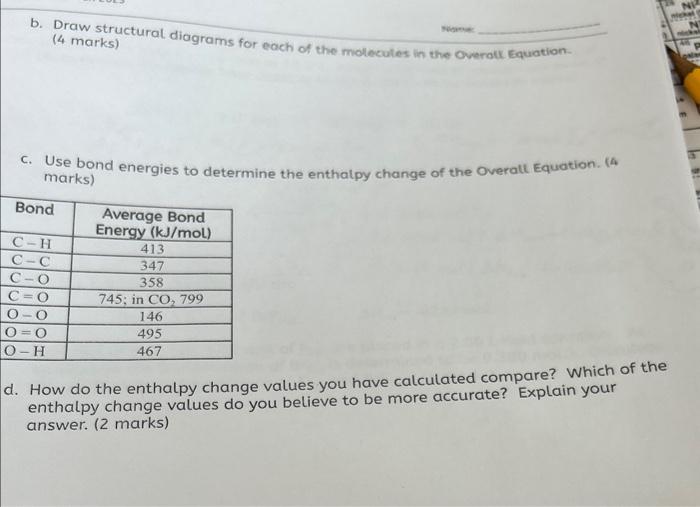
4. Bacteria can sour wine and beer by converting ethanol (C2H5OH) into ethanoic ac (CH3COOH) : Overall Equation: C2H5OH(1)+O2(g)CH3COOH(1)+H2O(1) The following enthalpy changes are determined using a bomb calorimeter. C2H5OH0)+3O2(g)2CO2(g)+3H2O(i)CH3COOH(1)+2O2(g)2CO2(g)+2H2O(1)Hcomb=1367kJHcomb=875kJ a. Use Hess's Law, and the complete combustion equations, to determine enthalpy change for the Overall Equation. (4 marks) b. Draw structural diagrams for each of the molecules in the Overoll Equation. (4 marks) c. Use bond energies to determine the enthatpy change of the Overall Equation. (4) marks) How do the enthalpy change values you have calculated compare? Which of the enthalpy change values do you believe to be more accurate? Explain your answer. (2 marks) 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


