Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Need Help? Read It Watch It Submit Answer [-/0.11 Points] DETAILS SCALCET9 2.7.053. MY NOTES ASK The quantity of oxygen that can dissolve in
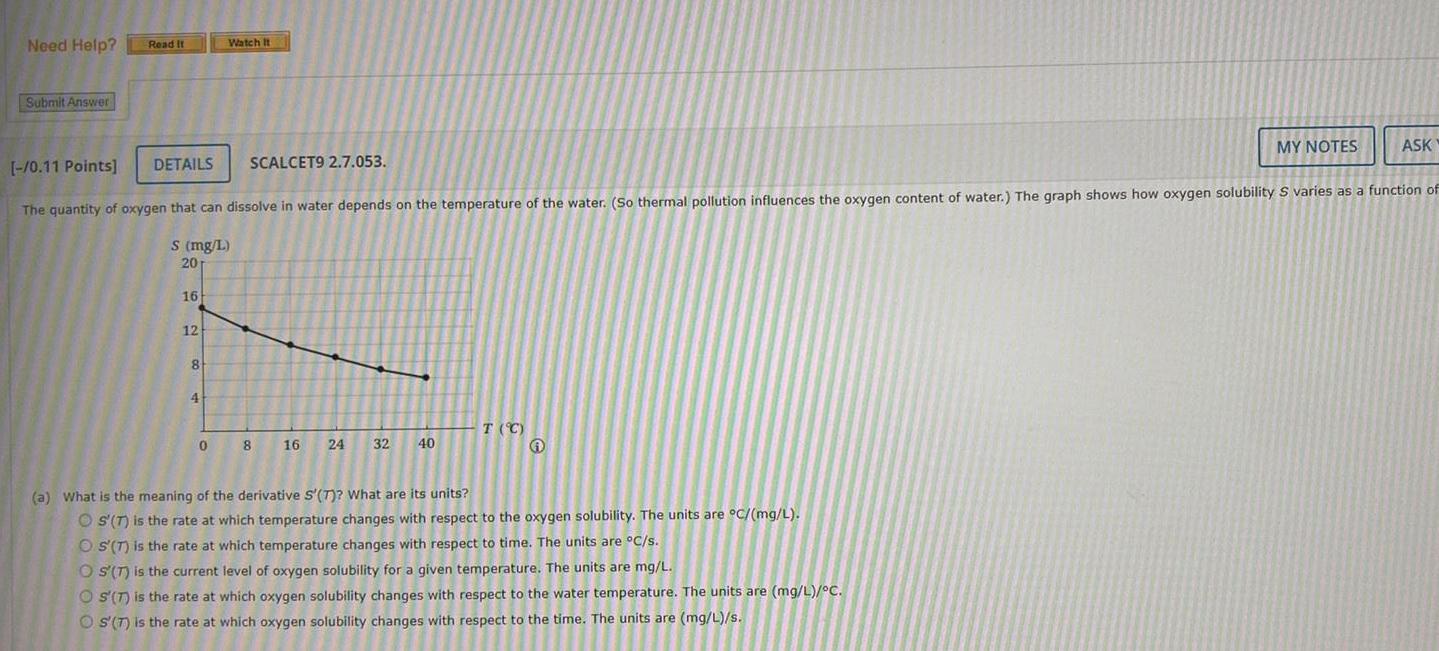
Need Help? Read It Watch It Submit Answer [-/0.11 Points] DETAILS SCALCET9 2.7.053. MY NOTES ASK The quantity of oxygen that can dissolve in water depends on the temperature of the water. (So thermal pollution influences the oxygen content of water.) The graph shows how oxygen solubility S varies as a function of S (mg/L) 20 16 12 8 4 T (C) 0 8 16 24 32 40 (a) What is the meaning of the derivative S'(T)? What are its units? OS'(T) is the rate at which temperature changes with respect to the oxygen solubility. The units are C/(mg/L). OS(T) is the rate at which temperature changes with respect to time. The units are C/s. O S'(T) is the current level of oxygen solubility for a given temperature. The units are mg/L. S'(T) is the rate at which oxygen solubility changes with respect to the water temperature. The units are (mg/L)/C. OS'(7) is the rate at which oxygen solubility changes with respect to the time. The units are (mg/L)/s.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


